In Vitro Differentiation of Human PBMC Derived Monocytes into M1 or M2 Macrophages in a Serum-free and Xeno-free Cell Culture Media
Macrophages are tissue-resident professional phagocytes and antigen-presenting cells (APC), which differentiate from circulating peripheral blood monocytes. They perform important active and regulatory functions in innate as well as adaptive immunity.1 Activated macrophages of different phenotypes are routinely classified into M1 macrophages (CAM) or M2 macrophages (AAM). The classically activated M1 macrophages comprise immune effector cells with an acute inflammatory phenotype. These are highly aggressive against bacteria and produce large amounts of lymphokines.2 The alternatively activated, anti-inflammatory M2 macrophages can be separated into at least three subgroups. These subtypes have various different functions, including regulation of immunity, maintenance of tolerance and tissue repair/ wound healing.1,2 Indeed, cells of the macrophage lineage exhibit extraordinary plasticity in response to endogenous as well as exogenous stimuli, which can allow conversion of the initial M1/M2 polarization processes2, for example M2-polarized macrophages can convert to the M1-activated status under certain conditions.
Primary human macrophages are difficult to isolate in sufficient amounts from tissue and do not proliferate in culture. In addition, it is commonly accepted that the obtained cells often exhibit significant phenotypical heterogeneity. Monocyte-derived macrophages provide an excellent alternative, since human blood monocytes are readily available in large numbers and can be differentiated into macrophages in vitro. The PromoCell Macrophage Generation Media were designed for the efficient differentiation of highly pure M1 or M2 macrophages directly from PBMCs as a starting material. The Macrophage Generation Media DXF are defined serum-free/xeno-free and provide a controlled culture environment devoid of all animal component-derived stimuli - a significant benefit in terms of monocytes and macrophages standing for highly reactive immune cells. As a result, these media lack unwanted non-defined and deleterious effects attributable to FBS and therefore enable standardized and controlled macrophage differentiation. In vitro differentiation of monocytes in the presence of the PromoCell M1-Macrophage Generation Medium DXF (C-28055, contains GM-CSF) leads to macrophages exhibiting M1-like polarized CD68+/ CD80+/CD163- macrophages, while the M2-Macrophage Generation Medium DXF (C-28056, contains M-CSF) promotes M2-like polarized CD68+/CD80-/CD163+ macrophages. The Macrophage Base Medium DXF (C-28057) represents the user-customizable version without cytokines and therefore needs appropriate supplementation by the user. If required, customized activation and subtype-specific polarization of the M1/ M2-polarized macrophages may be performed by the user.
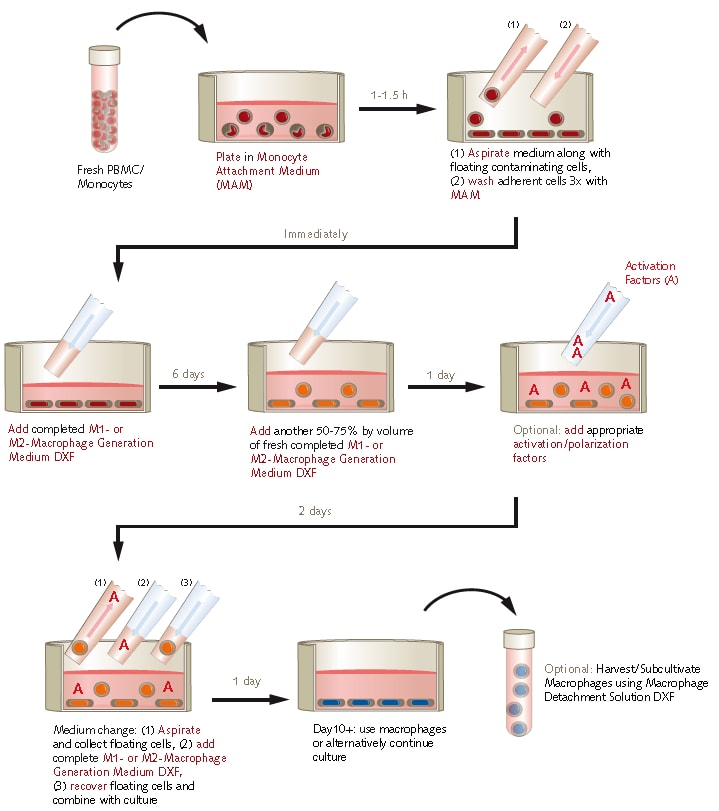
Figure 1. Protocol overview using PromoCell M1/M2 Macrophage Generation Medium DXF.
Macrophage Differentiation Protocol
- Isolate mononuclear cells (day 0). Isolate fresh PBMCs from buffy coats using your routine protocol.
- Analyze mononuclear cells (day 0). Count and analyze the isolated PBMCs for monocyte content, (e.g. using the FSC/SSC plot of a flow cytometer). Subsequently resuspend the cells at 100X106 PBMCs/ml in Monocyte Attachment Medium (C-28051).
- Let the monocytes attach (day 0). Plate freshly isolated PBMCs in an appropriate amount of Monocyte Attachment Medium (C-28051), e.g. 15 ml Medium per T-75 flask. Use a seeding density of 1 million/cm2 for Mononuclear Cells with a monocyte content of ≥25% and 1.5 million/cm2 for a monocyte content of <25%. Incubate for 1-1.5 hours at 5% CO2 and 37°C in the incubator without any further manipulation.
- Prepare the complete Macrophage Generation Medium DXF (day 0). Prepare the Macrophage Generation Medium DXF (C-28055, C-29056) by adding the thawed Supplement Mix aseptically to the Basal Medium. Swirl gently to obtain a homogeneous mixture. Then, add Cytokine Mix M1 or M2, respectively.
- Wash the adherent cell fraction (day 0). By vigorously swirling the tissue culture vessel loosen non-adherent cells and aspirate them. Wash the adherent cells, i.e. monocytes, three times with warm Monocyte Attachment Medium (C-28051) by swirling the vessel and aspirating the supernatant.
- Start the macrophage differentiation (day 0). Add an appropriate amount of complete M1- or M2-Macrophage Generation Medium DXF (C-28055, C-29056) to the cells, e.g. 20 ml per T-75 flask and incubate for 6 days at 37°C and 5% CO2 without medium change.
- Continue the differentiation process (day 6). Add another 50% to 75% by volume of fresh complete M1- or M2-Macrophage Generation Medium DXF (C-28055, C-29056) to the cells. Incubate the immature macrophages for another 3 days at 37°C and 5% CO2.
- Optional step: macrophage activation (day 7). For specific macrophage activation the whole volume of the culture is supplemented with adequate stimuli of the customers´ choice. Do not perform a medium change, just add the activation factors. Examples of macrophage activation by defined stimuli: Classically activated M1 macrophages can be generated by addition of IFN-γ (50 ng/ml) and LPS (10 ng/ml) to M1 macrophages. M2a-activation of M2 macrophages is achieved by 20 ng/ml IL-4. Supplementation with immune complexes and IL-1β or LPS will elicit M2b-activation, whilst IL-10, TGFβ or glucocorticoids lead to M2c-activation of M2-macrophages. An alternative type of M1 activated macrophage can be obtained by the activation of M2 macrophages with IFN-γ and LPS [2].
- Medium change (day 9). Aspirate the medium including suspension cells and collect it in a centrifugation tube. Immediately, pipet fresh complete PromoCell Macrophage Generation Medium DXF (C-28055, C-29056) supplemented with appropriate cytokines/activation factors to the cells. Centrifuge the cells in the tube for 15 min at 350 x g at room temperature. Discard the supernatant and carefully resuspend the cells in a small amount of fresh medium. Combine the resuspended cells in the tube with the adherent cells in the fresh medium contained in the tissue culture vessel. Incubate till the next day at 37°C and 5% CO2.
- The macrophages are ready (day 10). The macrophages may now be used directly in the plates where they reside, e.g. when performing phagocytosis assays. Maintenance of the culture for up to three weeks by performing weekly medium changes with fresh complete Macrophage Generation Medium DXF (C-28055, C-29056) is possible.
Results
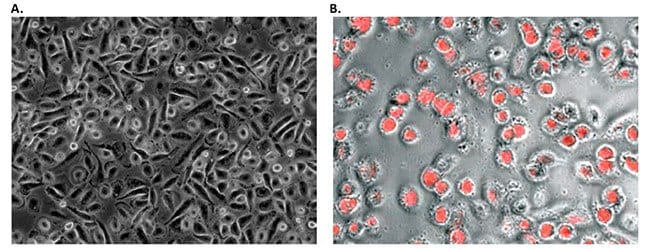
Figure 2. Morphology and phagocytic activity of human PBMC derived M1 macrophages. A) Phase bright images of M1 macrophages 24 hours after plating in PromoCell’s Macrophage Generation Medium DXF. B) PromoCell cryopreserved M1 macrophages ingest large numbers of fluorescently labeled E.coli. bacteria.
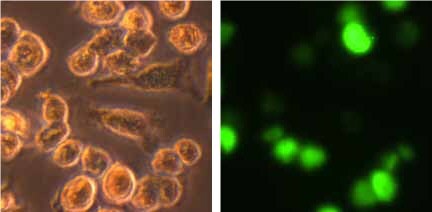
Figure 3. Morphology and phagocytic activity of human PBMC derived M2 macrophages. A) Phase bright images of M2 macrophages 24 hours after plating in PromoCell’s Macrophage Generation Medium DXF. B) PromoCell cryopreserved M2 macrophages ingest large numbers of fluorescently labeled E.coli. bacteria.
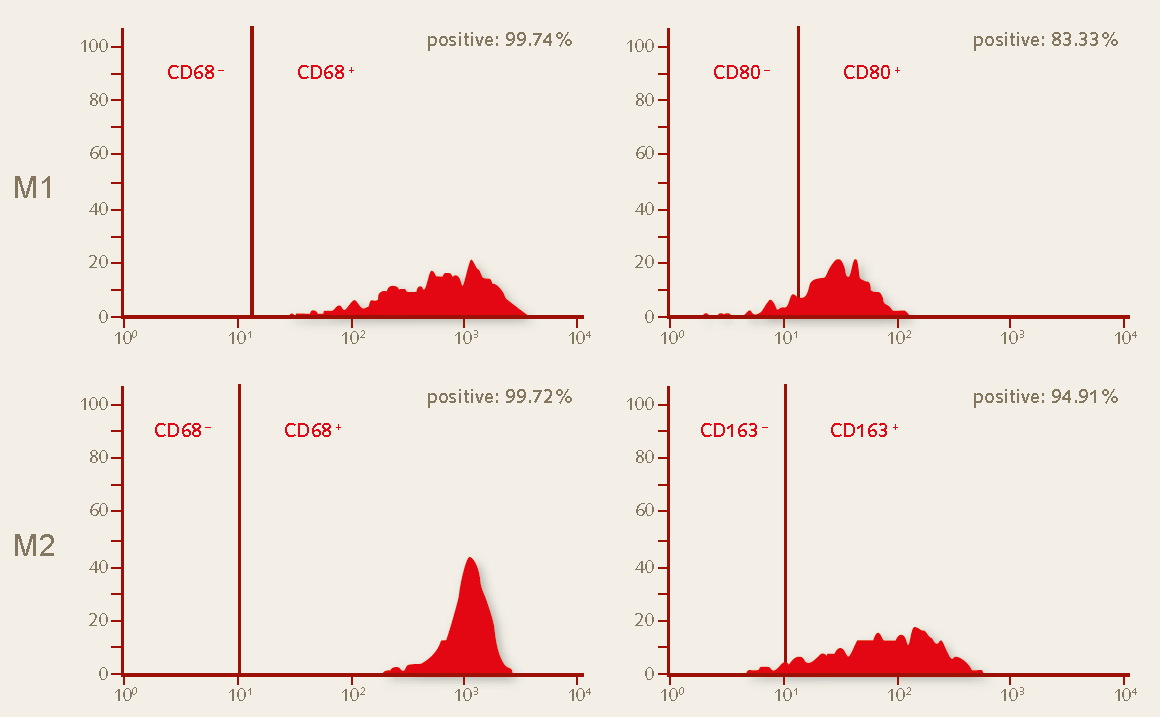
Figure 4. Flow cytometry analysis of day 10 M1 and M2 macrophages generated in the PromoCell Macrophage Generation Medium DXF. Fresh peripheral blood mononuclear cells (PBMCs) were plated in the Monocyte Attachment Medium. The purified monocytes were differentiated for 10 days without performing the optional activation step. M1 macrophages exhibit the CD68+ (99% positive) and CD80+ (83% positive) marker profile while M2 macrophages exhibit a CD68+ (99% positive) and CD163+ (95% positive) marker profile.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?