Although the previously described methods for Staudinger ligations work well even in biological environments, a modification forming a native amide bond without leaving the unnatural phosphine oxide moiety in the product would be more attractive yet. In 2000, the groups of Bertozzi and Raines simultaneously introduced alternative ligation strategies.1 Based on the same working principle as the nontraceless Staudinger Ligation the auxiliary phosphine reagent can be cleaved from the product after the ligation is completed leaving a native amide bond. Thus, the total chemical synthesis of proteins and glycopeptides is enabled overcoming the limitations of native chemical ligation (NCL) of a Cys residue at the ligation juncture.
Among the suitable phosphine reagents for traceless Staudinger ligations, diphenylphosphinemethanethiol (Figure 1), developed by Raines and co-workers, exhibits the best reactivity profile and has already found widespread application. This Raines ligation reagent is first acylated. Treatment with an azide leads to the formation of an aza-ylide. The nucleophilic nitrogen atom of the aza-ylide then attacks the carbonyl group, cleaving the thioester. Hydrolysis of the rearranged product finally produces a native amide and liberates the auxiliary as its phosphine(V) oxide (Scheme 6).2

Figure 1.
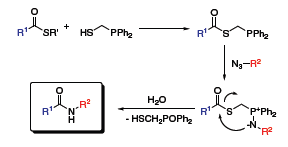
Scheme 6.
It’s recommended to use a freshly prepared Raines ligation reagent because it has only a limited stability. In this issue of ChemFiles, Sigma-Aldrich® proudly introduces new product 670359 as a shelf-stable, convenient source for this highly useful reagent (sold under license for research and development purposes only. U.S. Patent 6,974,884 and related patents apply). In the acetylthiomethyldiphenylphosphine borane complex 670359, the thiol and phosphine moiety are protected as acetyl ester and borane adduct, respectively. The active Raines ligation reagent can be liberated easily by treatment with DABCO® at 40 °C followed by basic ester cleavage (Scheme 7). Hackenberger and co-workers showed that acidic deprotection of the phosphine-borane was advantageous in glycopeptide and cyclopeptide preparations.3 In the latter case, a linear peptide with terminal azide and phosphine-borane groups was synthesized by SPPS. 95% TFA was used to deprotect the phosphine and the amino acid side chains simultaneously in a single step. Diisopropylethylamine (DIPEA) was then added to trigger the peptide macrocyclization by traceless Staudinger ligation, yielding cyclic Microcin J25 with 21 amino acids.

Scheme 7. (670359)
Other Staudinger ligation induced macrocyclizations have been published previously by Maarseveen and co-workers, who successfully used the Raines ligation reagent for the synthesis of a series of medium-sized lactams.4 Wong and co-workers reported the synthesis of 14 different glycopeptides through the traceless Staudinger Ligation.5 For this work, they also developed a protease-catalyzed method to selectively introduce an N-terminal azido group into an unprotected polypeptide, as it was needed for the subsequent ligation reaction.
Most recently, Raines and co-workers introduced a water-soluble variant of their reagent carrying dimethylamino groups (Figure 2). This substrate mediates the rapid ligation of equimolar substrates in water. In a pilot experiment, traceless Staudinger ligation was integrated with expressed protein ligation (EPL), revealing future opportunities in modern protein chemistry.6
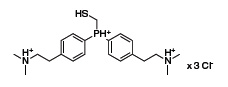
Figure 2.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?