Sulfur Dioxide in Beer
Photometric Determination of Sulfites with Ellman’s reagent

This Application Note demonstrates how the Spectroquant® Sulfite Test can be used to quantitatively determine sulfur dioxide in beer swiftly, efficiently, and reliably.
Sulfur dioxide or sulfite is contained in almost all types of beer. On the one hand, it occurs in beer as a natural result of yeast fermentation. On the other hand, it is also an agent used as an additive to preserve the beer and its raw ingredients, e.g. hops.1
Due to its characteristic property as a radical scavenger, the presence of SO2 prevents oxidative processes and also triggers the binding of carbonyl compounds, agents that cause a stale flavor in beer. Some people, however, react sensitively to SO2, showing symptoms such as nausea, vomiting, or headache.1 For this reason, some countries have defined limits that specify the maximum permissible concentration of SO2 in beer. In the EU, for example, according to Commission Regulation (EU) 2011/1129 the sulfur dioxide content may not exceed the limit of 20 mg/l SO2.2 Concentrations exceeding 10 mg/l SO2 must be indicated by the label “Contains sulfites”.3 The same mandatory declaration also applies in the USA.4
There are various analytical methods available to check the compliance with these limits. The European Brewery Convention (EBC) advocates a distillation method for quantification, which, however, involves a high investment of time, thus making swift analysis difficult.5 A further method for the measurement of sulfur dioxide in beer – one advocated by both the EBC and the American Society of Brewing Chemists (ASBC) – is the so-called p‑rosaniline method. The disadvantage of this method is that it uses toxic, in some cases carcinogenic substances, for instance p-rosaniline, formaldehyde, and mercury chloride.6,7
The Spectroquant® Sulfite Test using the photometric DTNB method offers a swift and precise alternative to the methods named above, with the additional advantage that it does not use any carcinogenic chemicals.
Sulfite Test Method
Reagents and Accessories |
|---|
Analytical approach
Sample preparation
Using the pH meter, adjust the pH of approx. 20 mL of fresh, cooled beer (4–8 °C) to 6.5–7.5 with sodium hydroxide solution. This operation should be carried out swiftly to avoid oxidation of SO2 with atmospheric oxygen. It is not necessary to bring the sample to a specific temperature before the analysis. To avoid incorrect results, check the sulfite content with the MQuant® Sulfite Test. Samples containing more than 60.0 mg/L SO32- must be diluted with water for analysis or distilled water.
Measurement
Zero adjustment:
It is recommended to zero the system each new working day using the same 10-mm rectangular cell that is used for measuring the sample and the sample blank. Please refer to the instrument manual for details on how to zero the system.
Sample blank:
The sample blank must be measured before performing the measurement of the sample itself. This step is necessary to compensate for the intrinsic color of the beer. Prepare the sample blank by mixing 8 mL of water for analysis and 2 mL of the beer sample, previously adjusted to pH 6.5–7.5. Transfer the mixture into a 10-mm rectangular cell bubble-free. Please refer to the instrument manual for details on how to measure the sample blank.
Measurement of the sample:
The sulfur dioxide concentration is measured with the Spectroquant® Sulfite Test as per the instructions of the package insert enclosed with the test kit. In deviation from the instructions given in the package insert, the reaction time here is 10 min. A shorter reaction time might cause losses. After this 10-minute period has expired, fill the measurement solution into the corresponding 10-mm rectangular cell bubble-free, place the cell in the measurement compartment, and start the measurement (where applicable).
After the measurement, the result can be read off from the instrument display. For easier data management und processing, transfer the results measured on the Prove spectrophotometers via Prove Connect to LIMS and/or Prove Connect to Dashboard. If the measurement solution is cloudy, the solution must be filtered through a syringe filter unit (cat. no. see above) after the reaction time has expired. Discard the first few drops of the filtrate.
Results
The results were verified by performing a reference analysis according to the ASBC Beer-21A method.1 In addition, spiking experiments were also performed, both for the measurement with the Spectroquant® test kit and for the measurement by the ASBC method. For this purpose, the sample was spiked with three different concentrations of sulfur dioxide (2, 5, and 10 mg/l SO2). Afterwards the recovery rate (RR) was calculated on the basis of the recovered content. The results for pale beers are shown in Table 1, those for dark and cloudy beers in Table 2.
Pale beers |
|---|
Dark and cloudy beers |
|---|
*According to ASCB the result should be reported to the nearest mg/L. However, for better comparison the result here is given with one decimal place.
Summary
The results yielded by the Spectroquant® test kit and those yielded by the p-rosaniline method of ASBC are in good agreement with each other. The deviations of the Spectroquant® Sulfite Test from the ASBC method range between -1.2 and 1.0 mg/L SO2. The mean deviation across all measurements is 0.4 mg/L SO2, a result that can be considered low.
In addition, spiking experiments were performed to assess the accuracy of the test kit. In pale beers, the measured recovery rate for the Spectroquant® test lay in the range 90–103%. In only two of the total of 15 spiking experiments was the error greater than 10%. In comparison, the ASBC method yielded clearly poorer results: in all light beers, the measured recovery rate of the ASBC method lies between 83–110%. In total, the error here was greater than 10% in six of the spiking tests, in other words in 40% of all samples. In the 2 mg/L spike of the “Kölsch“ beer, the recovery was even as low as 67%.
In the dark and cloudy beers, there were matrix effects to be seen in both methods, as indicated by the lower recovery rates. In these types of beer, the recovery rates shown by the Spectroquant® tests were on average 77–93%, those of the ASBC method 74–100%.
As a measure to exclude the possibility that the sulfur dioxide content in cloudy or dark beer types is judged too low, it is thus advisable to test the suitability of the test kit beforehand by a standard addition procedure (cf. the document “Identification of matrix effects by standard addition”), where necessary dividing the result by a correction factor.
The correction factor is calculated in the following way:
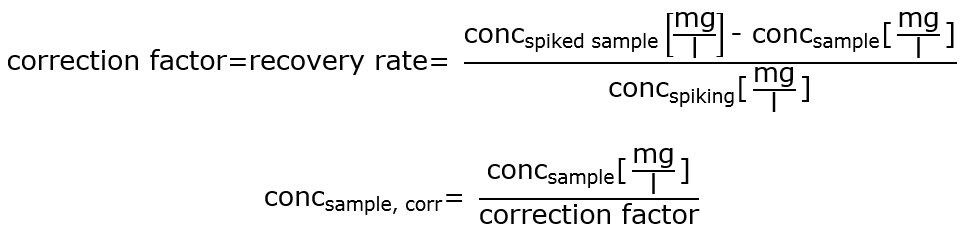
Note: The correction factor should only be used when the recovery rate lies within the same degree of magnitude over the entire spiked concentration range.
If this is not the case, the test kit is not suited for the analysis of the beer sample in question.
Conclusion
Spectroquant® Sulfite Test 101746 is a swift and precise method for the analysis of sulfur dioxide in beer. The detected sulfite concentrations are comparable with those yielded by the p-rosaniline method recommended by the ASBC. The spiking experiments carried out using the pale beer types even showed a substantially better accuracy for the Spectroquant® Sulfite Test in comparison with the ASBC method. Both methods exhibited matrix effects when applied to dark and cloudy beers.
In summary it can be stated that compared with the ASBC method the Spectroquant® Sulfite Test offers the following advantages:
- Higher accuracy in pale beers, comparable accuracy in dark and cloudy beers.
- Short analysis times thanks to the ready-to-use reagents and the elimination of the time-consuming calibration.
- Minimization of the potential health risk, since no carcinogenic chemicals are involved.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?