Annealing Oligonucleotides Protocol
This protocol is for annealing two single-stranded oligonucleotides with complementary sequences (Figure 1). Heating followed by cooling facilitates hybridization.
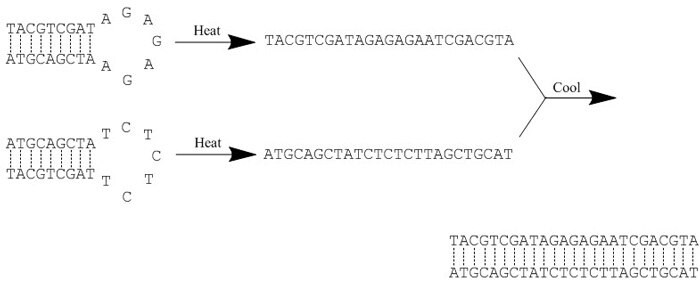
Figure 1.Example of an annealing reaction. Heat ‘breaks’ all hydrogen bonds, thereby disrupting any secondary structure within each oligonucleotide. Slow cooling then facilitates hybridization as new hydrogen bonds form between the complementary sequences.
Definitions / Abbreviations
EDTA: Ethylenediaminetetraacetic acid
NaCl: Sodium Chloride
Trizma® base: Brand name for Tris [Tris(hydroxymethyl)aminomethane]
Oligo: Abbreviation of oligonucleotide or oligomer. Oligonucleotides are short, single-stranded DNA or RNA molecules that must be annealed (heated or melted) so they can bond and form a double strand with an appropriate complementary DNA or RNA strand.
DNA annealing: This page discusses the annealing process for all oligonucleotides. Sometimes annealing is referred to as DNA annealing even though the process is used for RNA as well. Annealing is the process of heating and cooling two single-stranded oligonucleotides with complementary sequences. Heat breaks all hydrogen bonds, and cooling allows new bonds to form between the sequences.
DNA / RNA Annealing Equipment and Supplies
- Heat block or thermocycler
- 2 mL centrifuge tubes
- Pipette tips
- Milli-Q® H2O
- EDTA (Product No. E9884)
- NaCl (Product No. S3014)
- Trizma® base (Product No. 93362)
- Two single-stranded oligonucleotides with complementary sequences
DNA / RNA Annealing Method
The annealing process is divided into two main steps: 1) dissolution, and 2) annealing, either by heat block or thermocycler.
Oligo Dissolution
Although each oligonucleotide comes in a measured amount, for best results, verify it with a spectrophotometer to ensure that equal amounts of each oligonucleotide are added to the reaction.
- Dissolve each oligonucleotide in a volume of Annealing Buffer (see the buffer recipes below) so that each has the same concentration.
- The concentration of each oligonucleotide needs to be 2X the desired concentration of the duplex oligonucleotide.
Example
The desired concentration of the duplex oligonucleotide is 50 µM.
- Oligonucleotide 1: delivered with 10.55 OD (312.6 µg, 49.9 nmol); verify measured OD amount with spectrophotometer.
- Oligonucleotide 2: delivered with 9.04 OD (279.7 µg, 45.9 nmol); verify measured OD amount with spectrophotometer.
- Each oligonucleotide stock solution needs to be 2X the desired duplex oligonucleotide concentration, i.e. each stock solution needs to be 100 µM.
- For oligonucleotide 1, add 49.9 x 10 = 499 µL of Annealing Buffer to create a 100 µM stock solution.
- For oligonucleotide 2, add 45.9 x 10 = 459 µL of Annealing Buffer to create a 100 µM stock solution.
*This calculation is a shortcut that only works for creating 100 µM solutions and is used here for example purposes only. To learn more about calculating different oligonucleotide concentrations, see Handling Guidelines & Stability.
Oligo Annealing
Heat Block
- Mix equal volumes of the equimolar oligonucleotides in a microtube.
- Incubate the microtube at 95 °C for 5 min.
- Allow the microtube to slowly cool to room temperature (<60 min).
Thermocycler
Although a heat block will work, a thermocycler allows for a more consistent process.
- Mix equal volumes of the equimolar oligonucleotides in a PCR tube.
- Use the following thermal profile:
- Heat to 95 °C and maintain the temperature for 2 min.
- Cool to 25 °C over 45 min.
- Cool to 4 °C for temporary storage.
- Heat to 95 °C and maintain the temperature for 2 min.
- Centrifuge the PCR tube briefly to draw all moisture away from the lid.
Following deployment of the heat block or thermocycler, the duplex oligonucleotide is now ready to use or stored. To learn more about storing oligonucleotides, see Handling Guidelines & Stability.
Buffer Recipes for DNA Annealing
Make all buffers with Milli-Q® water.
Annealing Buffer Composition (1X)
- 10 mM Tris, pH 7.5 - 8.0
- 50 mM NaCl
- 1 mM EDTA
Ligase Buffer Composition (1X)
This buffer is typically used with T4 DNA Ligase.
- 50 mM Tris-HCl, pH 7.5
- 10 mM MgCl2
- 1 mM ATP
- 10 mM DTT
Kinase Buffer Composition (1X)
This buffer is typically used with T4 Polynucleotide Kinase.
- 70 mM Tris-HCl, pH 7.6
- 10 mM MgCl2
- 5 mM DTT
如要继续阅读,请登录或创建帐户。
暂无帐户?