Preparation of Monodisperse Polymer Spheres
Melissa A. Fierke
University of Minnesota
Material Matters 2008, 3.1, 13.
Monodisperse, surfactant-free polymer spheres for use as colloidal crystal templates can be easily obtained in reasonably large quantities. Typical synthesis methods for poly(methyl methacrylate) (PMMA) and poly(styrene) (PS) by emulsifier free emulsion polymerization are described below and yield spheres several hundred nanometers in diameter. Sphere sizes can be adjusted by altering the stirring rate, monomer concentration, reaction temperature, and amount of initiator. In both cases, the reaction is carried out in a four-necked round-bottom flask equipped with an electric stirrer with a Teflon stir blade, a water condenser, a pipet connected to a source of nitrogen, and a thermocouple probe (Figure 1a).
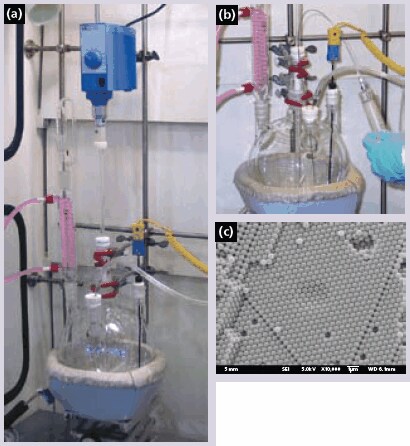
Figure 1.(a) Photograph of reactor setup for preparation of monodisperse polymer spheres. Please refer to the Product Table for list of reactor components. (b) Reagents (e.g. free-radical initiators) are added to the reaction via syringe without breaking N2 atmosphere inside the reactor. (c) SEM image of a typical PMMA colloidal crystal with multiple layers of monodisperse PMMA spheres packed in fcc structure. Point and line defects often observed in these colloidal crystals are also visible in the micrograph.
To synthesize PMMA spheres, 1600 mL of deionized water and 400 mL of methyl methacrylate (M55909) monomer are added to the flask. The mixture is stirred at approximately 350 rpm and bubbled with nitrogen. The temperature is increased to 70 °C and the system is allowed to equilibrate. After temperature stabilization, 1.50 g of the initiator, 2,2’-azobis (2-methylpropionamidine) dihydrochloride (440914), is dissolved in approximately 25 mL of deionized water and added to the flask (Figure 1b). Within several minutes, the mixture in the flask turns milky white. Over the course of the reaction (1–2 hours), the temperature rises several degrees before returning to 70 °C, signaling the end of the reaction.
The synthesis of PS spheres is very similar. 1700 mL of deionized water is added to the flask and heated to 70 °C while stirring at about 350 rpm. After the temperature has stabilized, 200 mL of washed styrene (240869) is added, and the temperature is allowed to equilibrate. Next, 0.663 g of the initiator, potassium persulfate (379824), is dissolved in 100 mL of water and heated to 70 °C before addition to the flask. The temperature is held constant, and the mixture is stirred for 28 hours.
After the polymerization reactions are complete, the polymer sphere solutions are filtered through glass wool to remove any large agglomerates from the solution. The spheres are then ordered into a colloidal crystal (Figure 1c) by one of several methods.
Sol-Gel Precursors
Sol-gel chemistry employing alkoxide, acetylacetonate (acac) and acetate precursors provides a convenient route to 3-dimensionally structured metal oxide materials. The chemistry is versatile both in terms of compatibility with all major nano- and micropatterning techniques described in this issue, as well as variety of available precursors, spanning the periodic table. The product table below groups sol-gel precursors by metal and can be used as a selection tool for synthesis of simple and mixed oxide materials. For example, titanium butoxide (244112) can be combined with barium acetate (255912) or lead acetate (398845) to make piezoelectrics BaTiO3 and PbTiO3.1,2
Oxalate Salts
Templated precipitation of metal salts offers an alternative to classical sol-gel chemistry for synthesis of 3D structured materials. Oxalates are metal salts of oxalic acid with general structure Mx(C2O4)y. Oxalates thermally decompose to give, depending on reaction conditions, structured metal oxides, carbonates, or metals, with gaseous (CO or CO2) reaction byproducts.3-6 Metal acetate hydrates (see Sol-Gel Precursors Table) can be converted to corresponding metal oxalates in-situ during the templating process, by treatment with a solution of oxalic acid.7
References
如要继续阅读,请登录或创建帐户。
暂无帐户?