Isolation and LC-MS Characterization of Illicit Bath Salts in Urine
Craig Aurand
Reporter US Volume 30.3
Introduction
With the rapid development of unregulated designer and synthetic compounds, the field of illicit drug testing has recently been met with a changing environment. Of most concern has been the development of a class of phenethylamine and cathinone compounds being marketed as “bath salts,” “transposed consumption,” “jewelry cleaner,” or “plant food.” Though sold as “not for human consumption,” these compounds are reported to generate stimulating effects similar to that of methamphetamine, heroin, and 3,4-methylenedioxymethamphetamine (MDMA also known as ecstasy)1,2. For a period of time, these compounds could be acquired legally through the internet and head shops due to a lack of direct legal control. In the US, both state and local governments have instituted bans on the sale of these bath salt compounds2. Forensic testing facilities often experience difficulty in testing these compounds due to the fact that they are not detected under normal ELISA testing methods; additional more specific LC-MS methods are necessary. The challenge for LC-MS detection of these particular bath salts resides in three sets of isobaric compounds, which require chromatographic resolution for positive confirmation and quantitation. For example, in Figure 1, both butylone and ethylone have the same monoisotopic mass, making these compounds indistinguishable when using time of flight mass spectrometry (TOF-MS).
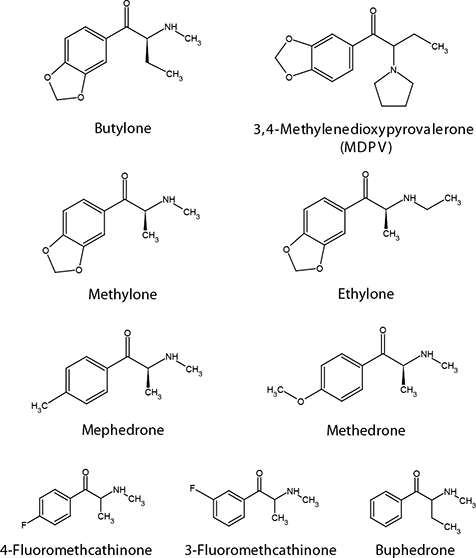
Figure 1.Structure of the Bath Salt Analytes
In this article, the analysis of bath salts from urine samples is demonstrated using polymeric solid phase extraction (SPE) sample preparation, followed by hydrophilic interaction liquid chromatography (HILIC) analysis with TOF-MS detection. HILIC conditions on the Ascentis Express HILIC (Si) phase are used for fast, high-resolution separation of nine synthetic bath salts. The polar basic nature of the bath salts makes these compounds prime subjects for HILIC separation. These compounds are difficult to retain on traditional reversed-phase C18 and even polar embedded stationary phases. Under reversed-phase conditions, high aqueous conditions are necessary to achieve retention, often leading to decreased ionization and detection in ESI-MS sources. High organic mobile phases are preferred for ESI-MS detection due to faster analyte desolvation and more efficient analyte ionization with their use. HILIC conditions not only enable increased retention of such polar basic compounds, but the high organic conditions also translate to increased response in ESI-MS. Figure 2 depicts the separation of all nine bath salts within six minutes on the Ascentis Express HILIC (Si) column.
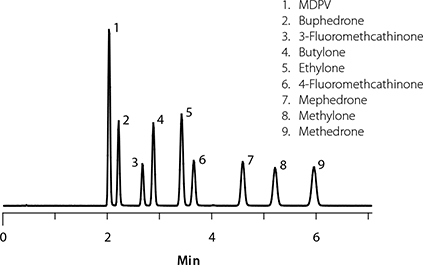
Figure 2.Analysis of Bath Salts on Ascentis Express HILIC (Si)
Conditions
- Column: Ascentis Express HILIC (Si), 10 cm x 2.1 mm, 2.7 µm (Product No. 53939-U)
- Mobile phase: (A) 5 mM ammonium formate acetonitrile; (B) 5 mM ammonium formate water; (98:2, A:B)
- Flow rate: 0.6 mL/min
- Pressure: 127 bar
- Column temp: 35 ºC
- Detector: MS, ESI(+), 100-1000 m/z
- Injection: 1 µL; sample: 200 ng/mL in acetonitrile
Supel™-Select SCX SPE was used for the processing and sample cleanup of the urine samples. The Supel-Select SCX is a polymeric cation exchange absorbent, containing a strong cation exchange sulfonic acid functionality. The selective retention of the bath salts is based upon the ion exchange mechanism between the anion functionality of the Supel-Select SCX and the basic functionality of the bath salts.
The strong ionic interaction with the analytes enables high organic wash solvents to be used for displacement of endogenous matrix, while maintaining retention of the analytes. Elution of the bath salts is achieved with the addition of a basic organic solvent. Only with these basic conditions are the bath salts eluted from the Supel™-Select SCX material. This approach results in a highly clean sample.
Experimental
Urine samples were collected from a volunteer donor and verified not to contain illicit bath salts. Control water samples, along with urine samples, were spiked to a level of 100 ng/mL with each target analyte making up the bath salt mixture. To ensure full ionization of the analytes, spiked samples were treated with formic acid to a final concentration of 0.1% formic acid. For comparison purposes, two separate sample prep techniques were performed prior to LC-MS analysis: SPE using the Supel- Select SCX and a “dilute and shoot” technique. The Supel-Select SCX SPE method is detailed in Table 1. For the dilute and shoot sample, 500 µL of spiked urine was mixed with 500 µL of acetonitrile and analyzed directly by LC-MS.
Sample Preparation |
|---|
Results and Discussion
Table 2 demonstrates the recovery of all nine bath salts for the control samples and spiked urine sample. Using the external calibration method, recoveries greater than 65% were observed for all analytes except MDPV (43.7%). Figure 3 illustrates the detection of bath salts in the spiked urine sample after SPE sample cleanup. Notice there are no interfering peaks in the chromatogram, demonstrating the effectiveness of the SPE sample cleanup. As a reference, Figure 3 also depicts the monitored bath salt ions for the blank urine sample. Again, there are no interfering peaks that could cause irregularities in analyte detection.
Common practice for preparation of urine samples is just dilution with mobile phase prior to analysis. For comparison, an acetonitrile-diluted urine sample was analyzed directly to show the difference in analyte detection. Figure 3 illustrates the monitored bath salt ions for the “dilute and shoot” urine sample. The anticipated peak response for the dilution sample prep technique should be half that of the SPE method, but notice in the chromatogram there are no distinguishable peaks in this sample. Not only is there a significant amount of matrix interference, but also the high aqueous content of the mobile phase has diminished the HILIC separation. Solvent mismatch between the chromatographic system and the injected sample should always be considered, regardless of HILIC or reversed-phase chromatographic modes of separation. Sample preparation using the Supel™-Select SCX not only allows for effective removal of interfering sample matrix, it also allows for easy analyte exchange into an organic solvent.
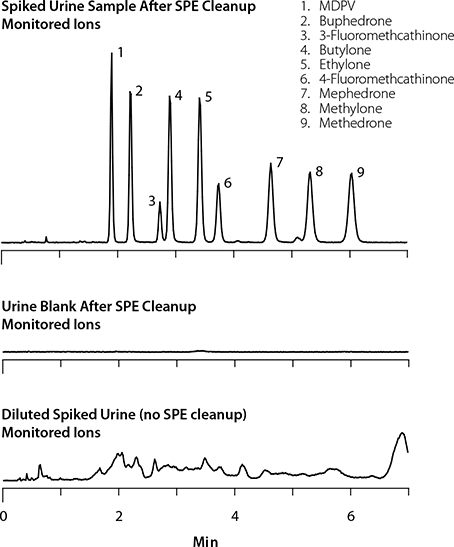
Figure 3.Spiked Urine Sample After SPE Cleanup; Urine Blank After SPE Cleanup; and Dilute and Shoot Spiked Urine (Monitored Ions)
Sample Preparation
- Sample/Matrix: 1 mL urine spiked to 100 ng/mL of bath salt mixture
- SPE tube: Supel-Select SCX, 30 mg/1 mL (Product No. 54240-U)
- Conditioning: 1 mL 1% formic acid acetonitrile, then 1 mL water
- Sample Addition: 1 mL spiked urine
- Washing: 1 mL water, 1 mL 1% formic acid in acetonitrile, 1 mL water
- Elution: 2 mL 10% ammonium hydroxide in acetonitrile
- Eluate post-treatment: thoroughly mix via vortex agitation, evaporate 1 mL aliquot to dryness, reconstitute in 100 µL water:methanol, 1:1
Conditions
- Column: Ascentis® Express HILIC (Si), 10 cm x 2.1 mm, 2.7 µm (Product No. 53939-U)
- Mobile phase: (A) 5 mM ammonium formate acetonitrile; (B) 5 mM ammonium formate water; (98:2, A:B)
- Flow rate: 0.6 mL/min
- Pressure: 127 bar
- Column temp: 35 ºC
- Detector: MS, ESI(+), 100-1000 m/z
- Injection: 1 µL; sample: 200 ng/mL in acetonitrile
Conclusion
The combination of using ion-exchange SPE with the HILIC HPLC separation provides a novel approach for the testing of problematic bath salt compounds. The isocratic Ascentis Express HILIC (Si) separation produces fast resolution of the isobaric compounds, thus, enabling the accurate quantitation of all nine bath salts. The Supel- Select SCX sample preparation method allows for efficient urine matrix removal while maintaining high analyte recovery. By utilizing ion-exchange mechanisms for sample cleanup, and taking advantage of the unique selectivity of chromatographic modes such as HILIC, analytical chemists can greatly improve the selectivity and sensitivity of their difficult bioanalytical applications.
Legal Information
Ascentis is a registered trademark of Sigma-Aldrich Co. LLC.
Supel is a trademark of Sigma-Aldrich Co. LLC.
如要继续阅读,请登录或创建帐户。
暂无帐户?