Improvement in LC/MS Analysis of Vitamin D Metabolites in Serum by Leveraging Column Selectivity and Effective Sample Prep
Clinical interest in vitamin D stems from its implications in an ever-widening range of human health conditions.1 This heightened interest has spawned the need for analytical strategies to assess an individual’s vitamin D status. Because LC-MS/MS has overcome many of the limitations of traditional immunoassay (e.g. cross-reactivity, matrix interferences), it is becoming more and more the analytical method of choice for certain clinical assays, including vitamin D. To maximize the effectiveness of LC-MS/MS experiments, it is important to consider factors that reduce measurement sensitivity and accuracy, restrict throughput and cause instrument downtime. This brief report presents a rapid and sensitive LC-MS/MS method for accurate determination of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3, and 3-epi-25-hydroxyvitamin D3 in serum.
Importance of Resolving Vitamin D Homologs
The metabolic pathways of vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) involve many compounds with varying degrees of biological activity and clinical interest. Chromatographic resolution of the various homologs of vitamin D2 is necessary for accurate quantitation, especially considering several key metabolites are isobaric and not distinguishable by MS alone. For example, separation of the active 25-hydroxyvitamin D3 from the 3-epi-25-hydroxyvitamin D3, whose biological role is currently unclear, may provide more accurate information for treatment and prevention.2 The aim of this study was to identify an HPLC stationary phase that would resolve vitamin D homologs, especially isobars, with short analysis time and high efficiency.
Sample Prep to Remove Interferences and Enhance MS Sensitivity
The hydrophobic character of vitamin D and its metabolites requires mobile phases with high concentrations of organic modifier, conditions that also elute endogenous interferences such as phospholipids. This phospholipid coelution causes ion suppression and/or enhancement in the MS instrument that reduces sensitivity and accuracy.3 Additionally, serum phospholipids and proteins foul HPLC and UHPLC columns and can cause instrument downtime. It is therefore important to remove them prior to analysis. In this study, commonly used protein precipitation and solid phase extraction methods were compared in terms of their ability to remove these interferences and improve detection accuracy.
Materials and Methods
- HPLC: Ascentis® Express F5, 10 cm x 2.1 mm I.D., 2.7 μM (Cat. No. 53569-U)
- Sample Prep Device: HybridSPE®-Phospholipid, 96-well plates, 50 mg/well (Cat. No. 575656-U)
- Standards: 25-Hydroxyvitamin D3 (Cat. No. H-083), 1α,25-Dihydroxyvitamin D2 (Cat. No. H-090); 3-epi-25-Hydroxyvitamin D3 (Cat. No. 705993)
- Protein Precipitation Method: Apply 100 μL of plasma to centrifuge vial followed by 300 μL of 1% formic acid in acetonitrile. Agitate via vortex for two minutes. Centrifuge 2.5 minutes at 15,000 rpm. Collect supernatant and analyze directly.
- HybridSPE®-Phospholipid 96-Well Method: Apply 100 μL of spiked plasma to the well, followed by 300 μL of 1% formic acid in acetonitrile. Agitate via vortex for four minutes, place on vacuum manifold and apply 10" Hg vacuum for four minutes. Collect filtrate and analyze directly.
Results
A pentafluorophenyl HPLC phase (Ascentis Express F5) was chosen because of its ability to rapidly resolve the vitamin D homologs tested, especially the 25-hydroxyvitamin D3 and the 3-epi-25-hydroxyvitamin D3 (Figure 1) that coelute on C18 stationary phases. Figure 2 shows the separation of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and 3-epi-25-hydroxyvitamin D3 on the Ascentis Express F5 column. Note that the coelution of 25-hydroxyvitamin D2 and 3-epi-25-hydroxyvitamin D3 is not an issue because they are resolved by the mass spec. Figure 3 shows the phospholipid monitoring chromatograms of coextracted matrix from standard protein precipitation and using the HybridSPE-Phospholipid technique. Comparing the sample prep methods, the simple and straight-forward HybridSPE-Phospholipid method was found to be far superior to standard protein precipitation. HybridSPE-Phospholipid selectively depleted the phospholipid matrix and precipitated proteins, providing no interference from the serum matrix. In contrast, the protein precipitation technique contained a large amount of coextracted phospholipid matrix resulting in interference that eluted in the retention range of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3, and 3-epi-25-hydroxyvitamin D3. This coelution reduces sensitivity and reproducibility, resulting in irregularities in quantitation as confirmed by the recovery and reproducibility data reported in Table 1.
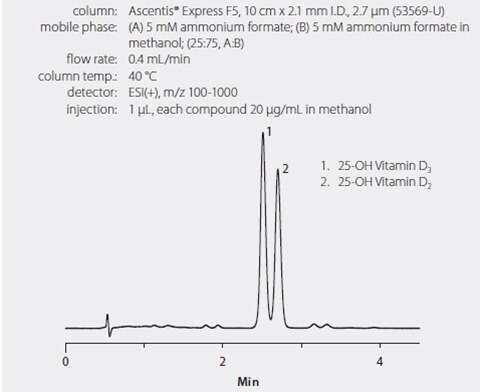
Figure 1. HPLC Separation of 25-Hydroxyvitamin D2 and 25-Hydroxyvitamin D3 on Ascentis Express F5
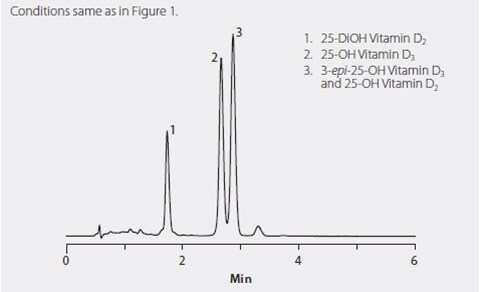
Figure 2. HPLC Separation of 25-Dihydroxyvitamin D2, 25-Hydroxyvitamin D3 and 3-epi-25-Hydroxyvitamin D3 on Ascentis Express F5
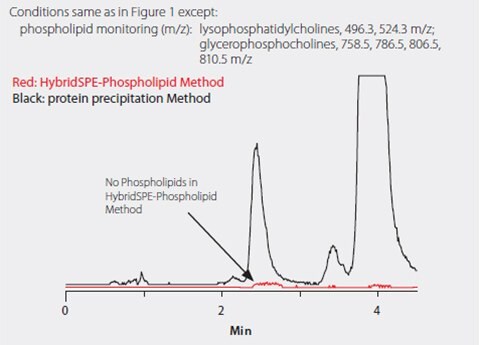
Figure 3. Serum Extracted Using HybridSPE-Phospholipid or Protein Precipitation
Conclusion
Chromatographic resolution of analytes still plays an important role in LC/MS applications; especially when dealing with isobaric compounds. The unique selectivity of the Ascentis Express F5 provided a fast and efficient method for the analysis of 25-hydroxyvitamin D and homologs from serum samples. The selective phospholipid depletion of the HybridSPE-Phospholipid method enabled an efficient sample cleanup increasing method reproducibility and accuracy. This approach demonstrates how selectivity, in both chromatographic and sample preparation, allows for efficient analysis that would otherwise be unattainable with traditional reversed-phase approaches. The combination of this novel sample prep technique along with the unique selectivity of the Ascentis Express F5 enables a fast and simplified bioanalytical method for associated vitamin D metabolites. For further reading and details of this method, please see reference2.
Our complete offering of solvents, reagents, columns, sample prep and other consumables to maximize speed and sensitivity in bioanalysis can be found here.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?