Molecularly Imprinted Polymer SPE Increases Sensitivity for the Extraction of Clenbuterol from Urine
Introduction
Clenbuterol (Figure 1) is a beta-agonist known for its growth-promoting properties in which use of the drug induces significant weight gain by increasing the proportion of muscle mass to fat. Although the US Food and Drug Administration, US Department of Agricultural and European Union have banned the use of clenbuterol for humans and livestock, illegal use of the drug still readily occurs1. For example, the drug is widely used among body builders and athletes due to its anabolic effects. Two Olympic athletes were banned from the 1992 Barcelona Olympic Games due to the drug, and two swimmers tested positive for the drug in the 2002 World Championships in Perth, Australia. Clenbuterol is readily used by farmers to give show animals a competitive advantage as well. In 1995, the US Department of Agriculture issued a press release stating that both they and the FDA will be taking enforcement actions against the use of clenbuterol2. In the 1990s, numerous outbreaks in clenbuterol food poisoning arose throughout Europe3. As recently as September 2006, over 300 cases of food poisoning occurred in Shanghai due to the consumption of clenbuterol contaminated pork4.
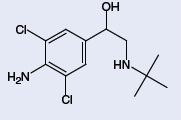
Figure 1: Structure of Clenbuterol
Due to the potential health risks and competitive advantage associated with clenbuterol’s use in livestocking and human performance enhancement, residue screening programs for the banned drug are conducted worldwide. It is therefore critical to develop a highly selective and sensitive analytical assay to monitor clenbuterol residues in difficult biological matrices such as urine, retina, tissues, etc.
In this report, we discuss the use of a molecular imprinted polymer technology (SupelMIP™) specifically designed for the selective extraction of trace levels of clenbuterol from urine for subsequent LC-MS-MS analysis. The technique was compared against a published method using a conventional hydrophilic polymer SPE phase5.
Molecular Imprinted Polymers
Molecular imprinted polymers (MIPs) are a class of highly cross-linked polymer-based molecular recognition elements engineered to bind one target compound or a class of structurally related compounds with high selectivity. Selectivity is introduced during MIP synthesis in which a template molecule, designed to mimic the analyte, guides the formation of specific cavities that are sterically and chemically complementary to the target analyte(s). As a result, multiple interactions (e.g., hydrogen bonding, ionic, Van der Waals, hydrophobic) can take place between the MIP cavity and analyte functional groups (Figure 2). The strong retention offered between a MIP phase and its target analyte(s) allows for the use of exhaustive wash procedures during solid phase extraction that results in superior sample cleanup prior to analysis.
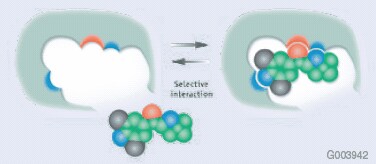
Figure 2:Visual Depiction of MIP Binding Site
Extraction of Clenbuterol from Urine
In this study, an extraction method using SupelMIP SPE – Clenbuterol was compared against a published method using conventional hydrophilic polymer SPE phase5. Table 1 describes the two extraction procedures.
SupelMIP SPE Offers Reduced Background Resulting in Greater Sensitivity
Clenbuterol was extracted from urine using both molecular imprinted polymer SPE and conventional hydrophilic polymer SPE via the procedures described in Table 1. Extracts were further analyzed via LC-MS-MS. From the LC-MS-MS chromatograms (MRM) depicted in Figure 3, we see that blank urine samples extracted with the SupelMIP protocol offered low background. In contrast, the conventional hydrophilic polymer SPE procedure co-extracted matrix interferences resulting in a high background response within LC elution area of clenbuterol (1-2 min.). This can potentially lead to lower assay reproducibility, accuracy and sensitivity thereby elevating lower limits of quantitation (LLOQ). Recovery for clenbuterol using the SupelMIP phase was 75% at the spike levels tested (0.1-1.0 ng/mL). 99% recovery was observed at the 0.1 ng/mL spike level. In contrast, reduced response levels were observed across the spike range tested using the hydrophilic polymer SPE protocol with a recovery of 8% at the lowest spike level (0.1 ng/mL). Note that both extraction procedures were repeated using buffer as a sample matrix, and recovery values were comparable for all three spike levels (data not shown); therefore, the lower response values observed for the polymer phase was primarily caused by matrix effects. To further demonstrate the selectivity and sensitivity differences between the two procedures, Figure 4 compares the linear relation of known spike concentrations vs. calculated concentrations determined from the signal responses obtained from blank urine extracts spiked post-extraction. Post-extraction spiked samples were compared for both the SupelMIP and polymer SPE protocols described. Increased levels of ion-suppression were observed for the polymer SPE protocol relative to the SupelMIP procedure.
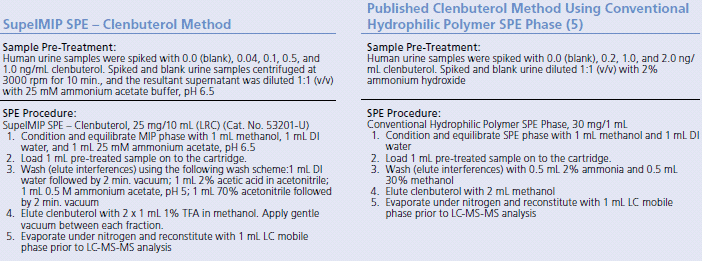
Table 1: Comparison of SupelMIP SPE – Clenbuterol Method and Conventional Hydrophilic Polymer SPE Method (53201-U)
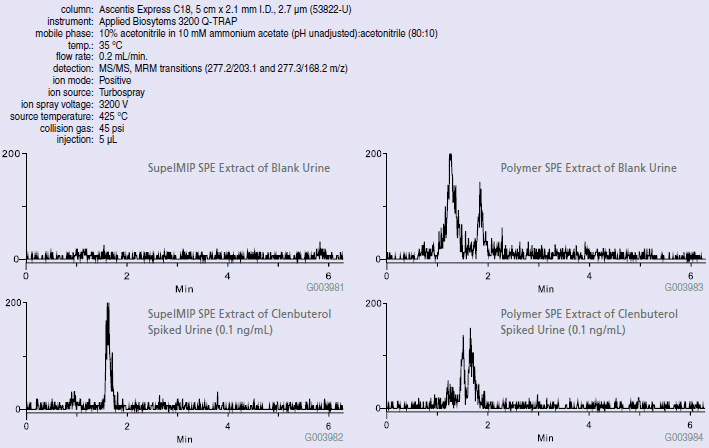
Figure 3: Clenbuterol Spiked Urine Samples Extracted with SupelMIP SPE vs. Convention Hydrophilic Polymer SPE (53822-U)
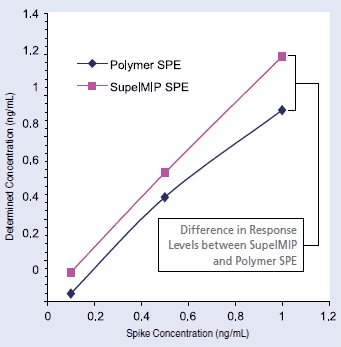
Figure 4:Known Spike Concentration vs. Determined Concentration for SupelMIP SPE and Polymer SPE Extracts of Clenbuterol from Urine (Post-Extraction Spike)
Conclusion
In this report, we demonstrated the extraction of SupelMIP SPE – Clenbuterol method against a published hydrophilic polymer SPE method for the trace extraction of clenbuterol from urine for subsequent LC-MS-MS analysis. Because selectivity is introduced during the development of the MIP itself, it allows for a binding site that is sterically and chemical complementary to the target analyte(s). The multiple interactions that take place between the imprint binding site and analyte(s) of interest offer strong interactions enabling the use of exhaustive wash conditions during the SPE process to provide cleaner extracts prior to analysis. This was demonstrated for clenbuterol in which the SupelMIP SPE approach offered greater selectivity resulting in lower limits of quantitation which is critical for the analysis of this banned drug.
Although the conventional hydrophilic polymer SPE method offers less procedural steps, sample prep selectivity was inadequate resulting in increased levels of background ultimately resulting in a less sensitive assay.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?