New Reagents for Selective Metalation, Deprotonation, and 1,2-Additions
Josephine Nakhla
Selective Metalations Using i-PrMgCl•LiCl and s-BuMgCl•LiCl
While halogen-metal exchange reactions are among the most common methods for preparing organometallic reagents, Li-halogen exchange reactions typically require low temperatures and offer limited compatibility with other functionalities. On the other hand, Mg-halogen exchange requires higher temperatures and the reagents are sometimes prone to elimination of HX (generating an olefin) because of their lower reactivity. Knochel and coworkers found the use of salt additives increased both the rate and the efficiency of the Mg-halogen exchange reaction. The most effective reagents were generated with R-MgCl (R = i-Pr, s-Bu) and 1.0 equiv of LiCl (termed TurboGrignards). The increased reactivity may be due to the breakup of polymeric aggregates known to exist in typical Grignard reagents as well as an increase in reactivity due to a negative charge on magnesium in i-PrMgCl2 -Li+. TurboGrignards allow the conversion of a variety of functionalized and highly sensitive substrates (including those containing functionalities such as CO2R, CN, OMe, and halogens) to their corresponding functionalized organometallic reagents, including both aryl- and heteroarylmagnesium derivatives. While rate enhancements are observed with the TurboGrignards, this increased reactivity does not have a negative impact on the overall scope of the reaction, permitting transformations to occur in the presence of a broad range of functional groups (Table 1).1
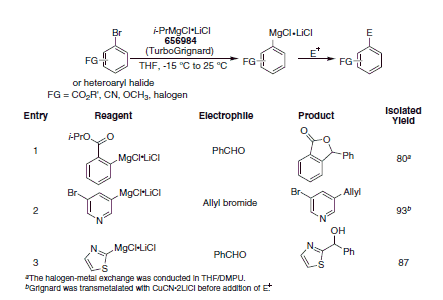
Table 1. Aryl/Heteroaryl Grignards prepared using i-PrMgCl•LiCl and reaction with electrophiles. (656984)
In summary, the advantages of the TurboGrignards include:
- Increased functional group compatibility
- Mild reaction conditions
- Convenient range of temperatures
- Side reactions inhibited
- Preparation of functionalized heteroaryl organometallics
- Large-scale production is feasible
Selective Deprotonations using Knochel-Hauser-Base
Deprotonation and functionalization of aromatics is a key synthetic transformation. However, common strong organic bases such as alkyllithiums or lithium amides cause competing addition reactions (Chichibabin reactions). Additionally, many amides must be generated in situ due to their low stability in solution. Finally, the low temperatures required when using these bases makes their use somewhat inconvenient. Knochel and coworkers have reported the use of 2,2,6,6-Tetramethylpiperidinylmagnesium chloride lithium chloride (TMPMgCl•LiCl) (Knochel-Hauser-Base) for regioselective deprotonation of arenes and heteroarenes. After further elaboration via addition of an electrophile, regioselective access to functionalized arenes and heteroarenes in excellent yields is achieved.3 TMPMgCl•LiCl offers a broadened reaction scope and tolerates a variety of functional groups, while preventing undesired side reactions (Table 2). The authors believe that oligomeric aggregates are broken up in the presence of TMPMgCl•LiCl.
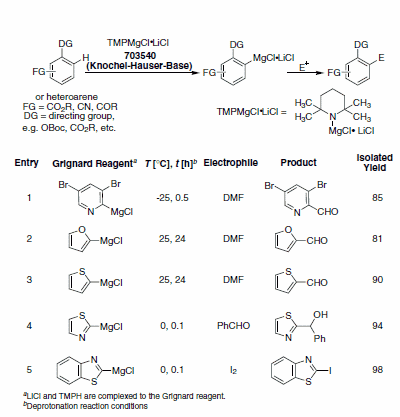
Table 2. Heteroaryl Grignards prepared using TMPMgCl•LiCl and reaction with electrophiles. (703540)
In summary, the advantages of the Knochel-Hauser-Base includes the following:
- High functional group tolerance
- High kinetic activity due to LiCl
- Regioselective metalation of arenes/heteroarenes
- No Chichibabin reactions
- Increased basicity
- Solubility in THF
Selective 1,2-Additions with LaCl3•2LiCl
Lanthanide salts have been shown to prevent competing reduction and enolization side reactions in the nucleophilic addition to ketones. However, both the solubility as well as the nature of the drying method of lanthanide complexes have historically limited the scope of their use. The addition of LiCl has resulted in enhanced reactivity of several organometallic reagents; thus, Knochel and coworkers prepared LaCl3•2LiCl and found it to be soluble in THF. In the presence of the oxophilic LaCl3•2LiCl, even sterically hindered and or enolizable ketones and Michael acceptors as well as unactivated imines can undergo 1,2-additions cleanly to provide the desired product resulting in a much improved reaction scope (Table 3).6
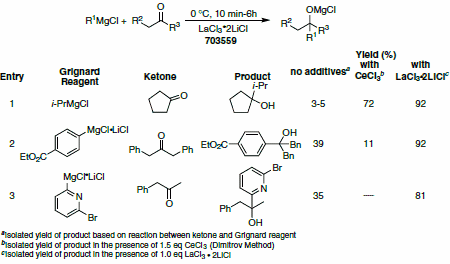
Table 3. LaCl3•2LiCl mediated addition to ketones. (703559)
Subsequent to the initial studies with the lanthanide salt- LiCl complexes, Knochel and coworkers reported that substoichiometric quantities of the lanthanide salt were sufficient to promote the desired 1,2-addition, as demonstrated in the addition of i-PrMgCl•2LiCl to unactivated imine derivatives (Scheme 2). This protocol was amenable to use of alkyl, aryl, and heteroaryl Grignard reagents.7
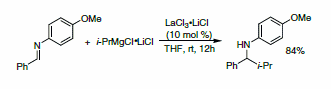
Scheme 2: 1,2-Addition of organomagnesium reagents in the presence of catalytic LaCl3•2LiCl
In summary, the advantages of LaCl3•2LiCl include the following:
- Low water content
- No pretreatment necessary
- Ease of handling
- Results in homogenous reactions
- Convenient reaction conditions
References
如要继续阅读,请登录或创建帐户。
暂无帐户?