Super Silyl Protecting Groups
Introduction
Tris(trimethylsilyl)silane (360716) (Figure 1) is commonly used as an alternative to tributylstannane (Bu3SnH) in radical reactions. Tris(trimethylsilyl)silane has been used in hydrosilylations,1 radical reactions,2 reductions of acid chlorides3 or carbon-halogen bonds,4 and hydrosilations of carbonyls.5 Another common application involves the use of the tris(trimethylsilyl)silyl (TTMSS, or super silyl) group when complexed with transition metals and main group elements.6
More recently, the super silyl group is being utilized in carbon–carbon bond forming reactions, where the researcher can take advantage of its unique reactivity that arises from its electronic properties and large steric bulk. Professor Hisashi Yamamoto has reported on the use of super silyl enol ethers in a variety of efficient one-pot sequential reactions.7

Figure 1
Representative Applications
Diastereoselective [2+2] Cyclizations
Boxer and Yamamoto have reported a diastereoselective [2 + 2] cyclization for aldehyde-derived silyl enol ethers containing the super silyl group. High yields and diastereoselectivity were obtained when the reaction was performed with a bulky aluminum catalyst. It would appear the transformation is unique to TTMSS protected enol ethers, as this [2 + 2] cyclization was unattainable with other typical silyl groups.1
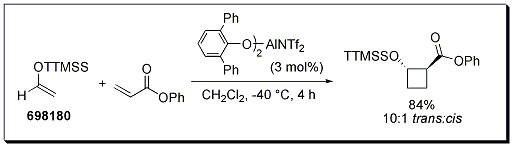
Scheme 1
Cross-Aldol Cascade Reaction
The super silyl group allows for the construction of β-hydroxy aldehydes for a broad range of aldehydes, providing 1:1 adducts in high yield.1 When a propionaldehyde-derived super silyl enol ether was used, syn selectivity was observed (Scheme 1, Table 1). This nicely complements the method previously reported by MacMillan that displayed anti selectivity.2
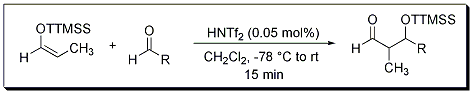
Scheme 1
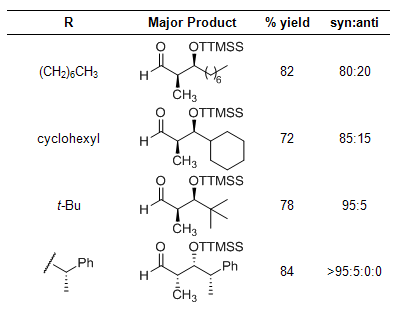
Table 1
The unprecedented reactivity and selectivity of the super silyl enol ether was extended to cascade reactions using 2.2 equiv of tris(trimethylsilyl)silyl vinyl ether, to provide the 2:1 adducts (Scheme 2, Table 2). Again, syn selectivity was observed for all reactions reported.
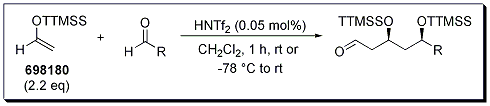
Scheme 2
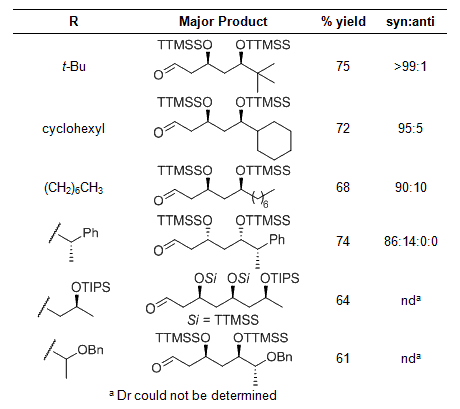
Table 2
Diastereoselective Sequential Reactions for Generating Polyols
The aldol reaction between a super silyl enol ether and an aldehyde can even be followed with a different nucleophile to generate various polyol architectures in a one-pot process. Silyl enol ethers, alkyl metal species, and even dienes for hetero-Diels–Alder reactions all were effective in the sequential reaction, and all reactions proceeded with high selectivity (Scheme 1, Table 1).1 When a (S)-2-phenylpropionaldehyde was employed, the reaction product was obtained in 97% ee, showing no appreciable racemization (entry 5).
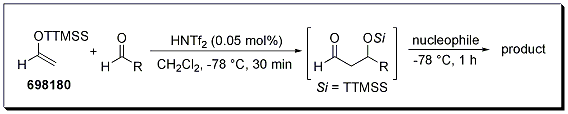
Scheme 1
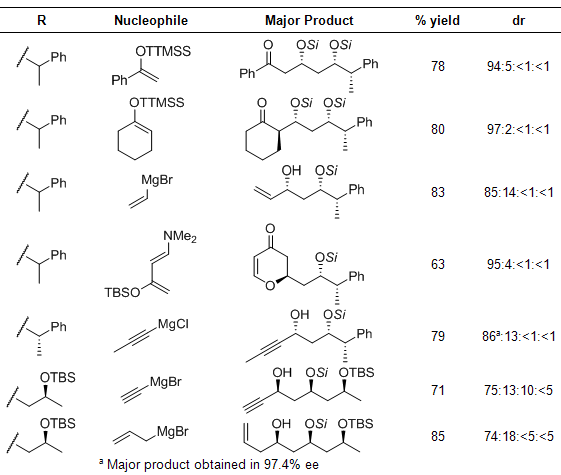
Table 1
This protocol can be extended to a sequential aldol-polyhalomethyllithium addition reaction, wherein the polyhalomethyllithium species is generated in situ by deprotonation of polyhalomethyls by LiTMP. These polyhalomethylcarbinols can be further transformed to the vinyl halide in good yields and excellent selectivity (Scheme 2).2
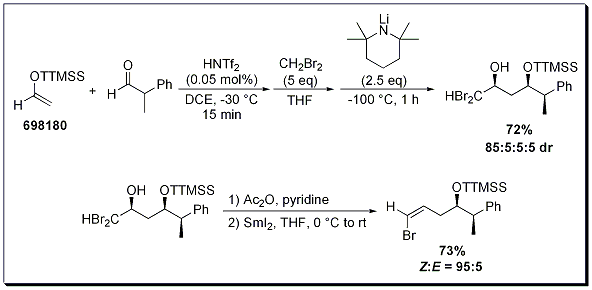
Scheme 2
Accessing Distinct Diastereomers and 4-Component Reactions
Varying the substrate choice in the one-pot sequential aldol-Grignard reaction gives the researcher the ability to construct distinct diastereomers. Depending on ketone super silyl ethers and Grignard reagent used, either the anti- or syn-1,3-diol can be generated in good yield and good to excellent diastereoselectivity (Scheme 1 and 2).
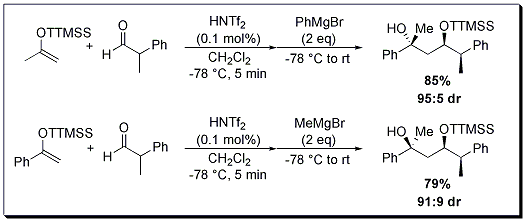
Scheme 1
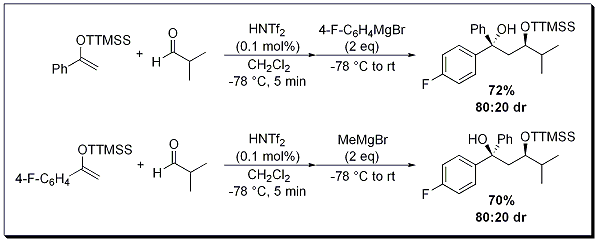
Scheme 2
Due to the excellent selectivity observed when using super silyl ethers, the 4-component, one-pot, sequential aldol-aldol-Grignard reaction was investigated (Scheme 3). Moderate yields and good diastereoselectivities were observed in the reported examples. The ability to selectively construct relatively complex molecules in one-pot make the super silyl group a very powerful tool in the synthetic chemists toolbox.
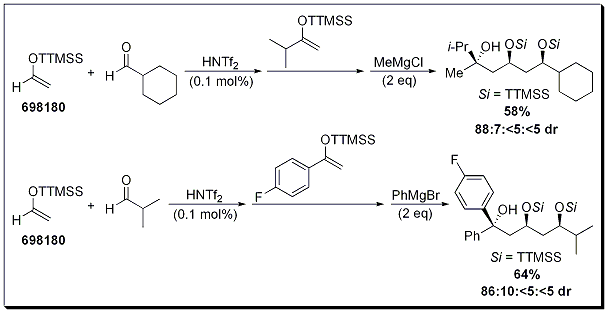
Scheme 3
References
如要继续阅读,请登录或创建帐户。
暂无帐户?