Extraction of Nitroimidazoles from Milk and Eggs using Molecularly Imprinted Polymers
Olga Shimelis1, Anna-Karin Wihlborg2, Marcus Rudolfsson2, Brian Boyd2, An Trinh1
1Supelco, Bellefonte, PA, USA, 2MIP Technologies AB, Lund, Sweden
Reporter US Volume 27.4
Introduction
Nitroimidazoles are anti-bacterial and anti-coccidial drugs used in the treatment of cattle, poultry, and pigs. These compounds and their metabolites are suspected carcinogens and mutagens. Consequently, their use in veterinary practice is strictly regulated within European Union Countries (Council Regulation 2377/90, Annex IV Banned Compounds).
Metronidazole (MNZ), ipronidazole (IPZ) and ronidazole (RNZ) belong to a list of pharmacologically active substances for which no maximum residue limit (MRL) can be fixed. As a result, their use is prohibited in food-producing animals, and any residue of these compounds detected in animals (and their products) intended for human consumption is considered a violation of EU regulation. The on-going surveillance of nitroimidazole drug residues and their metabolites in animal products requires a highly selective and sensitive assay for trace detection. In this report, a new molecularly imprinted polymer SPE phase (SupelMIP™) was evaluated for the extraction of nitroimidazoles: dimetridazole (DMZ), metronidazole (MNZ), ipronidazole (IPZ), ronidazole (RNZ), and their hydroxylated metabolites (DMZOH and MNZOH) (Figure 1). The nitroimidazole compounds were extracted from milk and egg samples and analyzed via LC-MS.
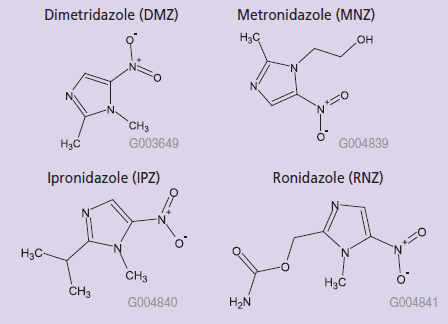
Figure 1. Structure of Nitroimidazoles
What are Molecularly Imprinted Polymers?
Molecularly imprinted polymers (MIPs) are a class of highly cross-linked polymer-based molecular recogition elements engineered to bind one specific target compound or a class of structurally related compounds with high selectivity. The MIP material is designed with cavities that are sterically and chemically complementary to the target analyte(s). As a result, multiple interactions (e.g., hydrogen bonding, ionic, Van der Waals, hydrophobic) can take place between the MIP captivity and the analyte.
SupelMIP SPE – Nitroimidazoles Extraction Procedure
One egg was homogenized. 10 g of the mix was spiked at levels of 1, 2, and 5 μg/kg nitroimidazoles: DMZ, IPZ, MNZ, RNZ, and metabolites DMZOH and MNZOH. Deuterated internal standards of each compound were added at the spike level of 2 μg/kg. For MNZ and MNZOH, DMZOH-d3 was used as the internal standard.
10 g of the spiked samples were extracted with 10 mL acetonitrile followed by centrifugation for 5 minutes. 2 g sodium chloride was mixed with the isolated supernatant followed by centrifugation. The extract was evaporated to dryness in a silanized glass test tube. The residue was reconstituted in 2 mL DI water and sonicated for 3 minutes prior to SPE processing.
Similar sample pre-treatment was followed for milk samples; however, the acetonitrile and sodium chloride extraction process was conducted in one step.
After sample pre-treatment, the resulting samples were processed using the SupelMIP SPE procedure described in Figure 2.
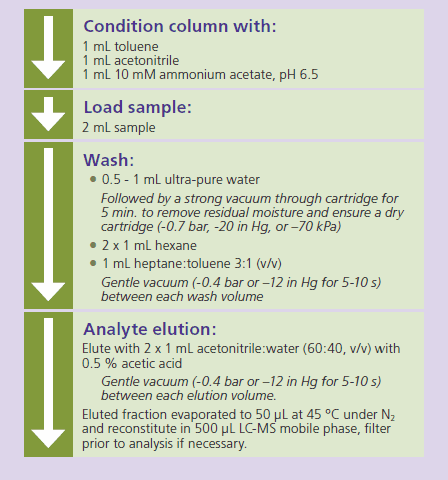
Figure 2. SupelMIP SPE - Nitroimidazoles Procedure
LC-MS Analysis
After SupelMIP SPE, the samples were analyzed via LC-MS using the conditions described in Table 1. Relative recovery was determined against internal standards for each of the spike levels tested. The data was also used to determine reproducibility and to estimate LLOD. Matrix (ionization) effects were assessed by spiking nitroimidazoles into post-SPE blank samples. The results were quantified against an external calibration curve diluted in buffer.
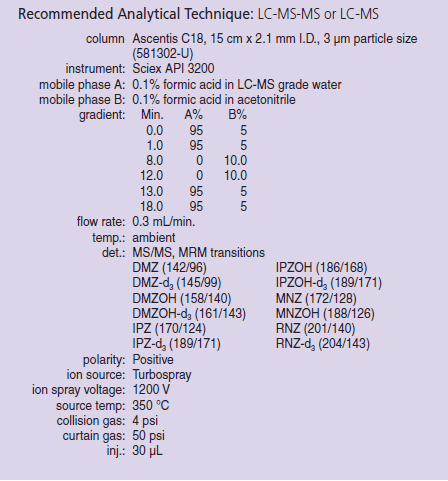
Table 1. LC-MS-MS Methodology (581302-U)
1Dueterated standard is available.
2Used DMZOH-d3 as an internal Standard.
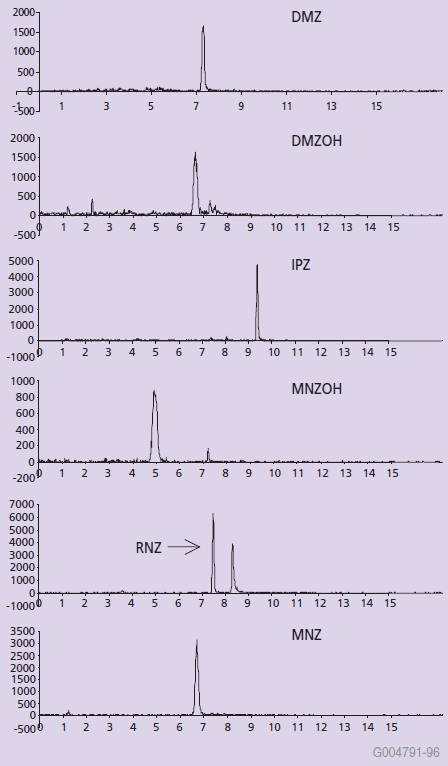
Figure 3. Ion-Chromatograms of 1 ng/g Nitroimidazoles Extracted from Eggs
Samples spiked into post-SPE blank extracts revealed good sample cleanup and low ion-suppression. The matrix ionization effects were minimal as evident from the 90-120% recovery values depicted in Table 3.
The estimated detection levels of nitroimidazoles ranged from 0.010-0.16 μg/kg in milk and 0.016-0.12 μg/kg in egg (Table 4). The LODs were estimated by analyzing the analyte and background response levels of the lowest spike concentration; and extrapolating a theoretic signal-to-noise ratio of 3:1.
Conclusion
In this report, SupelMIP SPE – Nitroimidazoles was evaluated for the extraction of nitroimidazoles and metabolites from milk and egg. Using molecularly imprinted polymer technology, high and reproducible recoveries were observed. Extraction was conducted with a high degree of selectivity minimizing ion-suppression, allowing researchers to achieve LODs at the ppt to low ppb levels. The SupelMIP method described in this report is highly robust and reproducible offering the necessary selectivity to achieve the stringent detection limit demands in food safety surveillance.
For more information, please visit our website: sigma-aldrich.com/supelmip
如要继续阅读,请登录或创建帐户。
暂无帐户?