SPE Cleanup of Wheat Extracts Prior to the HPLC Analysis of the Mycotoxin Deoxynivalenol
Ken G. Espenschied, Jennifer Claus
Reporter US Volume 31.3
Deoxynivalenol (DON) is a mycotoxin produced by the Fusarium fungal species graminearum and culmorum. These Fusarium fungi infect cereal crops primarily in wet conditions when temperatures range from 15–30 °C, resulting in grain head damage and DON contamination1. DON is the most common contaminant of grains and maize, and its presence may be a marker for other mycotoxins2. When ingested, DON inhibits protein synthesis through ribosomal binding and produces a strong emetic effect in some livestock animals and in humans3. The chronic exposure to DON in livestock may result in slowed growth, impaired immune function and reduced rates of reproduction, particularly in non-ruminants2,3. While no regulatory statutes are set in the United States, the FDA has issued an advisory for DON levels in various animal feedstuffs, with a suggested limit of 1 ppm in grain products used for human consumption. The European Union, however, regulates DON to levels as low as 0.2 ppm4,5.
Current methods for extraction and purification of matrix samples for DON analysis vary. Procedures using liquid-liquid partitioning, solid phase extraction (SPE), immunoaffinity columns (IAC), and multifunctional cleanup columns (MFC) are used. IAC and SPE methods are currently the most common ones used for sample cleanup in DON analytical protocols6. In this study, the extraction and cleanup method of the Supel™ Tox DON SPE cartridge was compared to that of a commercially available IAC column in order to determine differences pertaining to ease of use and process time. Wheat was chosen for extraction because wheat is a universal food staple that is routinely infected by Fusarium species and subsequently contaminated with DON7.
Experimental Overview
Three sample types were prepared; blank reference material, spiked reagent, and spiked reference material. Spiked reagent samples consisted of 84:16 acetonitrile:water for the SPE method and deionized water for the IAC method. All spiked samples (spiked reagent and spiked reference material) were prepared using a 100 µg/mL DON analytical standard. Actual DON concentration for the SPE samples processed was 2 µg/g. Actual DON concentration for IAC samples was 4 µg/g. Three replicates of each sample type were processed through each cleanup method using a vacuum manifold. Cleaned samples were then evaporated to dryness at 60 °C with 10–12 psi nitrogen stream before being reconstituted in 750 µL of mobile phase (92:4:4 water:acetonitrile:methanol). A single HPLC method was used to analyze samples prepared for both methods. Standards were prepared using 84:16 acetonitrile:water to match the mobile phase. Method conditions and a chromatogram of the 2 µg/g standard are shown in Figure 1. Initial testing was carried out using a 40 µL sample injection. Subsequent studies using the 10 cm x 2.1 mm, 2.7 µm Ascentis Express C18 column indicate a maximum 10 µL injection should be used to maintain good column efficiency and sample peak shape.
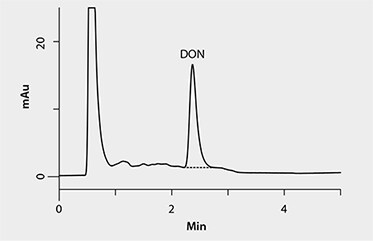
Figure 1.DON Standard (2 µg/g in mobile phase)
HPLC Conditions
column: Ascentis® Express C18, 10 cm x 2.1 mm I.D., 5 µm particles (50517-U); mobile phase: water:acetonitrile:methanol (92:4:4); flow rate: 0.400 mL/min; column temp.: 35 °C; detector: UV, 220 nm; injection: 40 µL
Results and Discussion
Sample Extraction
Overall time required to prepare extractions for the IAC and Supel Tox SPE samples was similar. However, filtration of the IAC extract presented difficulties that were not observed when filtering the Supel Tox SPE extract. Due to the binding requirements of IAC phase, the IAC extract was prepared using 100% water. When wheat flour was mixed with water, the presence of undenatured wheat proteins slowed the filtration process. Initial attempts to filter the IAC extract with 70 mm quantitative and 70 mm glass microfiber filter circles resulted in clogged filter media. It was necessary to switch to a 125 mm microfiber circle in order to filter the IAC extract. The SPE extract was prepared using 84% organic solvent and filtered quickly using a single 70 mm qualitative filter paper circle (Table 1).
Extract Cleanup
Fewer process steps are needed to complete the Supel™ Tox DON SPE cleanup when compared to the IAC method. The SPE method is a simple sample load and elute protocol requiring one reagent for the extract and the cleanup steps, and one set of glassware for sample collection. The IAC cleanup process involved more cartridge handling, more adjustment with closer monitoring of drop rates, an additional reagent for the wash step (phosphate buffered saline) and separate waste and sample collection glassware which require a mid-process vacuum manifold reconfiguration. The hands-on cleanup time needed to process samples using the IAC method was approximately three times greater than that of the SPE method (Table 2).
Matrix Removal and DON Recovery
Figure 2 is a set of representative chromatograms resulting from the analysis of blank and spiked reference material, along with a spiked reagent sample, that were processed using the Supel Tox DON SPE method. Matrix background levels were acceptable when using the SPE cartridge for the cleanup. DON recovery for both methods examined is summarized in Table 3. Recovery using the SPE method was >80%, and variation, expressed as an RSD value, was 2%. Variability and recovery values using the Supel Tox DON SPE method fell well within acceptable limits at the concentration tested8.
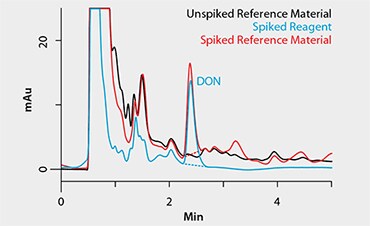
Figure 2.Supel Tox SPE Method Chromatograms
Conclusion
Supel Tox DON SPE cartridges were successfully used to clean wheat matrix samples for subsequent HPLC analysis of DON. The cartridge performed competitively when compared to an IAC column cleanup and analysis when using the same reference material. The Supel Tox DON SPE cleanup required fewer process steps and used fewer reagents than is typical for IAC cleanup methods9. In addition the wheat extract produced in the Supel Tox DON extract was more easily filtered than that from the IAC process due to the use of organic in the extraction solvent. Overall process time for both methods was similar; however, the Supel Tox SPE hands-on cleanup time was significantly shorter and the process steps were simpler when compared to the IAC method. It is expected, therefore, that the SPE method will be more readily transferrable to laboratory staff and less vulnerable to sample processing errors. Supel Tox DON SPE offers an easier to use alternative to IAC cleanup cartridges in routine testing applications.
Legal Information
Ascentis is a registered trademark of Sigma-Aldrich Co. LLC
Supel is a trademark of Sigma-Aldrich Co. LLC
References
如要继续阅读,请登录或创建帐户。
暂无帐户?