Mesoporous Materials: Properties and Applications
Introduction
Materials with specially ordered porous features on the nano-scale have important applications in optics, catalysis, drug delivery systems, coatings, cosmetics, bio-separation, diagnostics, gas-separation, and nanotechnology. Nanoporous materials consist of an amorphous or crystalline framework with void spaces, which may be cylindrical or cage-type. Most nanoporous materials fall under three major categories: microporous, mesoporous, and macroporous.1
Microporous materials — such as MOFs, zeolites, carbons, and amorphous glasses — exhibit extremely narrow pore size distributions in the range of 0.5 – 2 nm.2 These materials can display high thermal stability and catalytic activity useful in cracking processes and can also serve as ion exchange media, drying agents, and gas separation materials. Metal organic frameworks (MOFs) are one of the fast growing classes of microporous solids.3 Zeolites and related crystalline molecular sieves have an intrinsic limit on their pore dimension and accessibility owing to the pore templates available for their synthesis. In contrast, macroporous materials with pores sizes between 50 and 1000 nm, such as porous polymer beads, allow for easy access to the internal pores at the cost of selectivity. These drawbacks lead to the development of mesoporous materials, which have an intermediate pore size range, between 2 – 50 nm.4
Mesoporous materials have a number of key advantages:
- Narrow pore size distributions and high surface areas (>500 m2/g).
- Framework/wall substitutions with various metal oxides (MO2) including silica, alumina, and titania
- Simple functionalization strategies with organics
- Biocompatibility and low toxicity.
Structural Properties and Characterization of Mesoporous Materials
Ordered mesoporous materials can be classified according to their structure dimensions, and pore geometry, e.g., either (2D- or 3D-) cylindrical or (3D-) cage-type structures. Cylindrical structures, such as MCM-48, AMS-6 (Iad), MCM-41, SBA-15, and NFM-1 (p6mm), possess uniform pore diameter and show potential towards applications in catalysis, adsorption, and as drug delivery vehicles. In contrast, cage-type mesocaged solids, such as FDU-1(Imm), SBA-1(Pmn), and AMS-8(Fdm) consist of spherical or ellipsoidal cages that are connected three-dimensionally by smaller cage-connecting windows, which could be used to control the mass transfer of active agents.
Characterization methods including powder x-ray diffraction, N2 adsorption/desorption, scanning electron microscopy (SEM), and transmission electron microscopy (TEM) are routinely used to elucidate the structural and textural properties of mesoporous materials such as silica (Figure 1).

Figure 1. Scanning Electron Microscopy image of a typical mesoporous silica material.
Powder X-ray diffraction (XRD) is commonly used to identify the crystallographic symmetry of material phases at both the nano- and meso-scale. Phase identification for mesoporous materials from powder X-ray diffraction can be difficult because most of the peaks appear at low angles and may overlap because of similar short-range order. Figure 2 shows a typical result of the morphology and structural order of the pores within mesoporous silica. Detailed analysis of pore order and symmetry at the mesoscale can be achieved using high resolution transmission electron microscopy (HRTEM). HRTEM characterization is crucial for extracting detailed information at these length scales.
Gas adsorption is a complementary method used to obtain a comprehensive characterization of porous materials. Adsorption of gases at various relative pressures on the porous solids provides information about properties including surface area, pore volume, and pore size. Figure 3 shows a typical gas absorption isotherm.
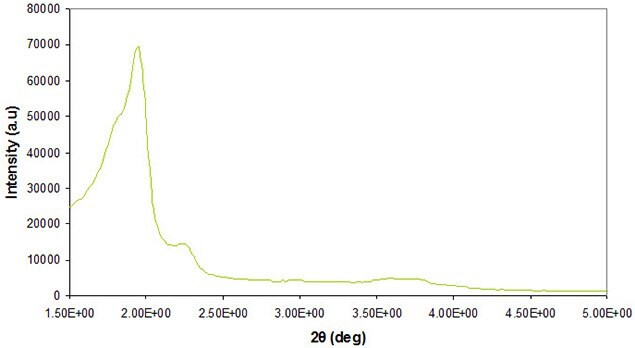
Figure 2. X-ray diffraction of a typical cubic mesoporous silica material.
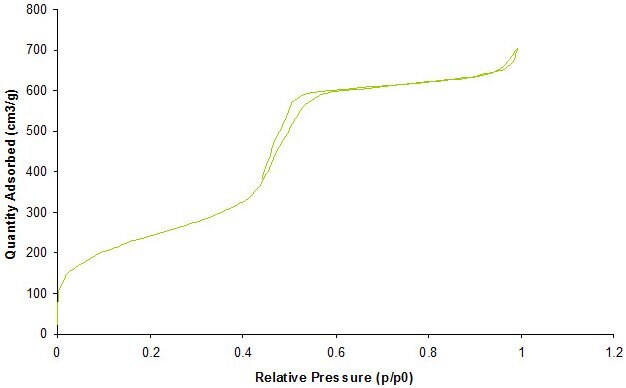
Figure 3. Representative characterization of mesoporous silica nanoparticles by N2-Isotherm corresponding to a surface area 850 m2/g with a pore size of 3.8nM.
Functional Mesoporous Silicas
One of the most versatile classes of mesoporous materials are porous silicas, which combine well defined pore sizes with the known biocompatibility of silica in a range of applications. An important property of a porous silica material is its ability to incorporate functional organics (R) on the silica wall. Porous silica possesses a high density of surface silanol (≡Si-OH) groups after calcination. The reaction of these silanols with various silanes presents different functional groups (≡Si-R) on the silica framework, which can be used to conjugate molecules of interest.5
Properties and Potential Applications of Functionalized Materials
- Encapsulation of pharmaceutical drugs, proteins, and other biological molecules
- Adsorbent for gases, ions, and molecules
- Loading of active sites for catalysis
- Loading of nanoparticles including iron oxide, gold, etc.
We offer nanoporous Si modified with three different functional groups: Propylamino, Propylcarboxylic Acid, and Propylthiol groups (Table 1).
Applications of Nanoporous Materials in Research and Industry
Drug Delivery Systems
The main challenge in the development of drug delivery systems (DDS) is that drug efficacy diminishes before reaching the target, primarily due to the excretion of the drug from the body. In addition, the drug carrier must be non-toxic and inert during the treatment period. Because most biological molecules and pharmaceuticals are on the order of a few nanometers, nanoporous silica with a pore size of 2 – 30 nm is of great relevance for such life science applications.6
Catalysis
In catalysis, high surface area materials with nanoscale features are used to develop highly selective catalysts that reduce energy use and waste/pollutant generation in industrial applications.7 Porous materials, such as zeolites (microporous solids), are widely used in industry as catalysts and catalyst supports. However, when large molecules are involved in a catalyzed reaction, mass transfer limits the suitability of zeolite structures. Attempts to improve the diffusion of reactants to catalytic sites have been resolved by enlarging pore sizes to the meso range8. These ultra-selective catalysts can cut costs significantly in many industries.
Diagnostics
Mesoporous materials are ideal in diagnostics applications due to their increased image contrast and chemical stability. Moreover, functional moieties that can be conjugated within the pores enables new possibilities for multiple measurements and detection. Due to the low toxicity of silica based porous materials and their ability to host a variety of fluorescent markers, dyes and drugs can be used to track the location of therapeutic agents and their activity.
Adsorbents
The high surface area of nanoporous materials allows their use as adsorbents for various gases, liquids, and toxic heavy metals. The uptake of these substances can be increased significantly based on the surface properties (hydrophobicity, hydrophilicity, or functionality), of the mesoporous silica materials. Several applications, such as removal of pollutants from water, storage, of gases (e.g., CO2, H2, O2, CH4, H2S), adsorptive xylene separation, and separation of biological and pharmaceutical compounds, have been addressed through the use of mesoporous materials as adsorbents.
Chromatography
The large pore volume, surface area, and narrow pore-size distribution of mesoporous silica, makes it a good candidate for size exclusion chromatography. These materials have been proposed as supports or stationary phases for size exclusion chromatography, capillary gas chromatography, proteomics separations, normal phase High Pressure Liquid Chromatography (HPLC), as well as enantioselective HPLC.
Our Mesoporous Materials
We offer a wide range of porous materials including nanoporous silica, nanoporous alumina, porous carbon, and functional nanoporous silica materials. In addition, fluorescent labeled nanoporous silica particles designed particularly at diagnostics and pharmaceutical applications are now available.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?