Evaluation of Irradiation Influence of Culture Media on Growth Promotion
Comparative analysis of half-Fraser Readybag® with non-gamma irradiated half-Fraser GranuCult™
Listeria monocytogenes is one of the most dominant foodborne pathogens worldwide. EN ISO 11290-1:1196 + Amd 1:2004 and EN ISO/DIS 11290-1:2014 describe the test method for the detection and enumeration of Listeria monocytogenes. The selective primary enrichment broth to be used is called half-Fraser broth, which contains half of the concentration of acriflavine and nalidixic acid in comparison to Fraser broth.
Half-Fraser GranuCult™ and Readybag® culture media are EN ISO 11290-1:1196 + Amd 1:2004 and EN ISO/DIS 11290-1:2014 compliant. Both types of media are quality controlled by an ISO 17025 accredited laboratory. GranuCult™ is granulated and supplied in 500 g packs. Readybag® is also a granulated culture media, but provided in prefilled, gamma irradiated pouches of 12.5 g or 62.0 g. The required size depends on the detection method. Granulated culture media leads to easier and faster media preparation, preventing clumping and reducing airborne toxic and allergenic dust.
After autoclaving of half-Fraser GranuCult™, the addition of selective agents is necessary. For the Readybag® pouches the need for upfront media preparation is eliminated and no supplement handling is necessary. The food testing routine is simplified because sterile water is simply added before use.
The aim of the comparison study was to demonstrate that gammairradiation (10-20 kGy) has no influence on the growth promotion of Listeria monocytogenes. Half-Fraser GranuCult™ was used as a reference of a non-gamma-irradiated dehydrated culture media.
The evaluation was performed by the Institute of Veterinary Food Science – Department of Veterinary Medicine, Justus Liebig University of Giessen, Germany, utilizing growth promotion tests and food trials.
Method |
|---|
All products were obtained from Merck KGaA, Darmstadt, Germany and Merck Life Science GmbH, Eppelheim, Germany.
Growth Promotion Test
Incubation at 30 °C (± 1) for 24 h (± 2) for the primary enrichment was chosen according to ISO 11290. Afterwards half-Fraser broth can be refrigerated before transfer to the second enrichment (Fraser broth) for a maximum of 72 h (see section 9.3.2 EN ISO/DIS 11290-1:2014).
The shortest (22 h) and longest (26 h) incubation time were selected to evaluate the final concentration of colony forming units (cfu) per sample. In addition, bacterial counts (cfu/mL) were calculated after cold storage as described above. Growth promotion data were collected for all four tests strains (Table 2) and repeated 10 times.
The initial inoculation level (cfu per sample) was determined by plating out on Tryptic Soy Agar (TSA, Figure 1).
Readybag® and GranuCult™ half-Fraser media were inoculated with the same suspension to ensure comparability and the tests were performed in parallel.
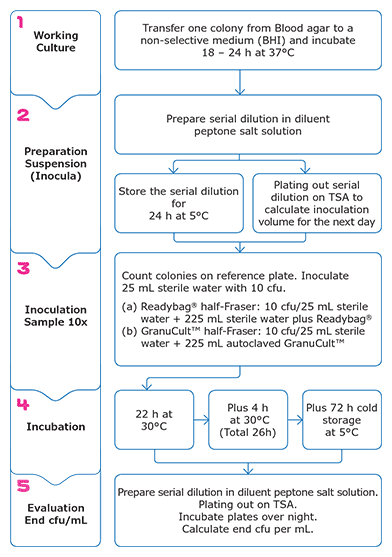
Figure 1. Workflow of Growth Promotion Test for Listeria. The growth promotion test was repeated 10 times per strain. Inoculation level was around 10 cfu.
Food Trials
Cooked prawns and cream cheese were selected as food matrices to evaluate growth promotion with matrix. Spiking was conducted as described above using the two Listeria monocytogenes food isolates. PALCAM agar was chosen as second selective culture media. The cold room storage step of 72 h was also included in the food test procedure.
For comparison, inoculation and detection of Listeria monocytogenes in one food matrix was performed in parallel with Readybag® and GranuCult™ culture media using the same bacterial suspension. A total of 20 food samples were inoculated (10 samples per week).
The food matrices were inoculated with 1 colony forming unit per sample and the level was controlled by plating out on TSA (cfu/sample).
Singlepath® L'mono was used for confirmation after the first enrichment. This step was done once per sample and plating medium after 24 h agar plate incubation. A well isolated, suspect colony was picked from the selective plate and re-suspended in 250 μL Fraser. The sample was incubated for 1 h at 37 °C and cooled down to room temperature. Test performance and interpretation was carried out as described in the pack insert of Singlepath® L'mono.
For each, trial one negative control and one positive control (102/25 g) were included. All food samples were not naturally contaminated.
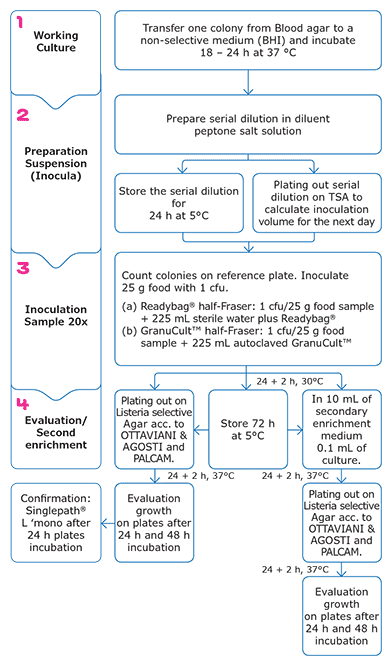
Figure 2. Workflow of food trials - Listeria monocytogenes (Food Isolates). Step 1 and 2 similar to the workflow for the Growth Promotion Test. First enrichment = half-Fraser and second enrichment = Fraser.
Results
Growth Promotion Test
The results of the growth promotion tests are shown in Figures 3 to 6. Growth curves are based on the median values of 10 results per test strain and sampling time point. The 25th percentile (lower quartile) and 75th percentile (upper quartile) are marked.
All samples showed growth of target bacteria. The slope of the curves between 22 h and 26 h incubation is similar. That means that the rate of growth is equal. The 25th and 75th percentile of both growth curves are overlaying in most cases.
The growth promotion test confirmed that half-Fraser can be refrigerated before transfer to the second enrichment as a reduction of growth was not detected. In all samples an increase of bacteria growth was measurable.
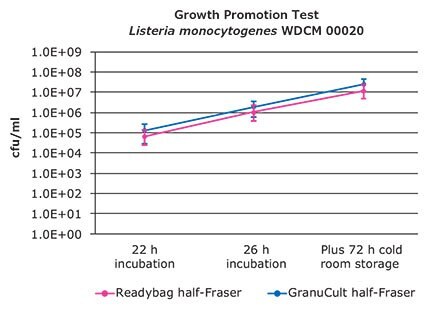
Figure 3. Colony forming units after 22, 26 h incubation and 72 h cold room storage of Listeria monocytogenes WDCM 00020.
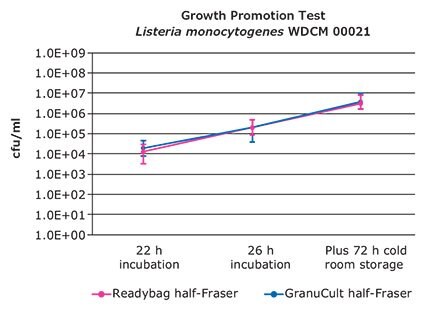
Figure 4. Colony forming units after 22, 26 h incubation plus 72 h cold room storage of Listeria monocytogenes WDCM 00021.
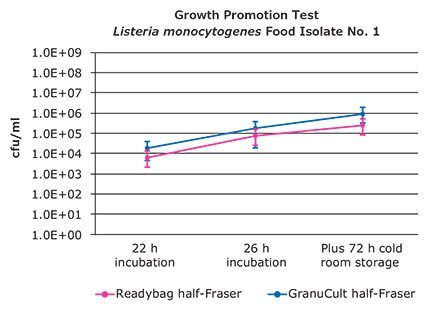
Figure 5. Colony forming units after 22, 26 h incubation plus 72 h cold room storage of Listeria monocytogenes. Food Isolate No. 1.

Figure 6. Colony forming units after 22, 26 h incubation plus 72 h cold room storage of Listeria monocytogenes. Food Isolate No. 2.
Food Trials
The results of the food sample testing (n=20) are summarized in Table 3 and Table 4. The table shows the positive and negative results of culture media evaluation after 24 h plate incubation (ALOA and PALCAM) and the sensitivity of Singlepath® L‘mono.
The detection rate of positive and negative samples is in line with those obtained from the secondary enrichment and the cold room storage.
The food samples were inoculated with 1 colony forming unit and approximately 50% test samples were positive.
Comparison of the frequency of positive samples using the Fisher exact test gives a P-value of 0.75. Therefore the observed difference in number of positive samples is not statistically significant.
Interpretation
This external evaluation study investigated the potential impact of gamma irradiation on growth promotion by using four different Listeria monocytogenes strains including two food isolates.
GranuCult™ half-Fraser and irradiated Readybag® half-Fraser culture media were inoculated at low levels with 10 cfu. EN ISO 11290-1:1196 + Amd 1:2004 and EN ISO/DIS 11290-1:2014 state a time frame of 24 h (± 2) for the primary incubation step. After 22 h and even after 26 h of incubation both products show a similar rate of growth. The slope of the growth curves is equal. The currently revised ISO version now also includes the opportunity for cold storage of the primary enrichment for up to 3 days before further processing. The study revealed no detrimental effects on the growth promotion for Listeria monocytogenes. However, cfu/mL slightly increased as Listeria are known to grow at low temperatures (psychotropic).
The food trials using cooked prawns and cream cheese at a low level spiking of 1 cfu/25 g showed also comparable results considering the additional storage period of 3 days according to EN ISO/DIS 11290- 1:2014. This included also a confirmation step with Singlepath® L‘mono.
The gamma-irradiation of Readybag® has no significant negative influence on growth promotion. This was documented by growth promotion tests and food trials. GranuCult™ half-Fraser and irradiated Readybag® half-Fraser culture media can be used for Listeria detection according to EN ISO 11290-1:1196 + Amd 1:2004 and EN ISO/DIS 11290-1:2014.
Literature
- ISO International Standardisation Organisation: Microbiology of food and animal feeding stuffs – Horizontal method for the detection and enumeration of Listeria monocytogenes - Part 1: Detection method - Amendment 1: Modification of the isolation media and the haemolysis test, and inclusion of precision data. EN ISO 11290- 1:1996 + Amd 1:2004.
- ISO International Standardisation Organisation: Microbiology of the food chain – Horizontal method for the detection and enumeration of Listeria monocytogenes and other Listeria spp. - Part 1: Detection method. EN ISO/DIS 11290:2014.
如要继续阅读,请登录或创建帐户。
暂无帐户?