Performance Comparison and Evaluation of Microbiological Recovery of Aspergillus Brasiliensis Using Ez-fit® Filtration Units
This study shows the performance of EZ-Fit® Filtration Units in comparison to seven competitors focused on Aspergillus brasiliensis ATCC® 16404 according to international standards (Pha.Eur.2.6.12 Harm. with USP (1231 chapter 61); JP (16G (4.4.2)).1,2,3 This study was designed to demonstrate the recovery rates and to observe and record general handling during study execution.
With the combination of ease of handling and optimal microbial recovery rate, this study demonstrated the advantages of EZ-Fit® Filtration Units. In addition to the recovery rate reliability, the fast read-out of 48 hours with Aspergillus brasiliensis ATCC® 16404 shows how our membrane filters could also save time.
During the manufacturing and purification steps of a protein, or while a beverage is clarified and stabilized prior to bottling, the quantity of micro-organisms has to be monitored. The standard method is membrane filtration, followed by incubation on culture media.
The EZ-Fit® Filtration Unit is a disposable filtration device for bioburden testing of liquid samples such as water, process samples, or final products. It is designed for optimizing and securing the laboratory workflow to provide time-saving and reliable microbiological results. Two different EZ-Fit® Filtration units are available, the blue unit with absorbent pad and the pink unit without absorbent pad to cover a wide range of applications, offering a selection of membranes and the flexibility to use liquid or solid culture media.
The pink EZ-Fit® Filtration Unit has a completely new drainage design, allowing faster filtration of turbid samples, leading to a decrease in the filtration time of up to 40% in comparison to the blue EZ-Fit® filtration unit. The absence of pad also limits potential inhibition concerns when testing antibiotics or other pharmaceutical samples.
Fungal presence in manufacturing and cleanroom areas is a serious concern in the pharmaceutical and food & beverage industry, which could lead to product recall and damage to brand image. Final products and raw materials are at risk, therefore regulatory bodies such as the FDA or local pharmacopeias advise carrying out continuous monitoring.
Aspergillus brasiliensis ATCC® 16404 is a commonly used mold for performing validation and growth promotion studies in the pharmaceutical industry, and was therefore selected for this comparative study.
Standards1,2,3,4,5 specifies Tryptic Soy Agar (TSA) for total aerobic microbial and Sabouraud Dextrose Agar (SDA) for total yeast and mold counts. EZ-Fit® Filtration Units and a range of competitive products were therefore tested on TSA and SDA. Brands from Japan, China, Latin America, India, USA, and Europe were included in this study to cover the global membrane filter availability on the market.
Material & Equipment

Figure 1.EZ-Fit® Manifold, 3-place

Figure 2.EZ-Stream®-Pump
The filtration devices tested had the pore size of 0.45 μm. Membrane filters were used with sterile single-use plastic funnels.
Additional materials were used according to standard recommendations.
Method
- The microbiological recovery rate was evaluated using the microorganism specified in Pha. Eur. 2.6.12 Harm. with USP (1231 chapter 61) and JP (16 G (4.4.2)).1,2,3,4
- Samples were prepared by adding 10 mL sterile water to each funnel, followed by the addition of 100 μL inoculum containing 10–100 CFU / 100 μL, and finally adding sterile water to a total sample volume of 100 mL.
- Following filtration, membranes were immediately transferred to either TSA or SDA agar plates, and incubated according to standard recommendations.1,2,3
- Membrane filtration was conducted in triplicate, and the mean CFU was calculated for each filtration unit.
- Typical colony forming units (CFU) were counted when clearly visible and easy to identify.
- The mean CFU for each membrane filtration was compared to spread plate controls in order to calculate recovery.
- The product recovery (PR%) was determined using the method specified in ISO 7704:1985 with the following formula:5
PR = NS/N0
NS = colony forming units on membrone filter
N0 = colony forming units on spread plate
Handling
There are several general handling aspects where EZ-Fit® Filtration Unit can be considered an improvement over the competition.
- Lids of the EZ-Fit® Filtration Units remain intact during transport, thereby reducing contamination risk.
- The legible 360° graduation, the shape of the funnel and the clear funnel material make it easy to observe the sample during the filling and the filtration process and to visualize the filtration endpoint.
- The EZ-Fit™ Filtration Units fit perfectly onto the filter heads, whereas those of the competitor products require a rubber stopper and suction which risks leakage.
- Easy single-handed removal of the funnel, with no filter damage. Easy removal of the membrane and very smooth application onto the agar prevents the formation of bubbles under the filter.
- Grids are clearly visible. Small colonies can be seen for simple identification.
- The device design ensures that complete sample volume is delivered to the membrane surface preventing any leaks or sample by-passes.
- Both blue and pink EZ-Fit® Filtration Units (with and without pad) can be used in combination with the other EZ-Family members and with our culture media, available in ready-to-use or dehydrated formats.
Results
Figures 3 and 4
illustrate the calculated recovery rates on TSA and SDA agar respectively. Recovery of each organism was compared across eight different membrane filters. The line marks a recovery rate of 70%.
Qualification of new methods is typically performed according to USP <1227> Validation of Microbial Recovery,4 which states that the average number of CFU recovered should be at least 70% of the control.
EZ-Fit® Filtration Units (with and without pad) and EZ-Pak Filter show a steady recovery rate of over 70% on both SDA-4% and TSA in all four filtration rounds. Brand A-G reach the 70% only with SDA agar, but on TSA varying results are documented.

Figure 3.Recovery of Aspergillus brasiliensis ATCC® 16404 on SDA-4%-Agar
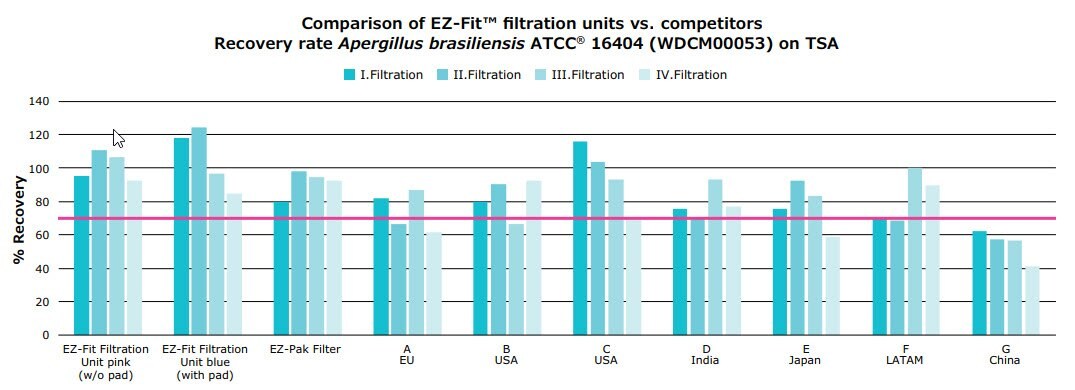
Figure 4.Recovery of Aspergillus brasiliensis ATCC® 16404 on TSA
The innovatively designed EZ-Fit® Filtration Unit membrane allows detection of Aspergillus brasiliensis ATCC® 16404 after 48 hours (Figure 5). The number of colonies did not change in comparison to a longer incubation time (up to 7 days). After a 72 hour incubation, the blackening of the colonies appeared.
The earlier evaluation and blackening of Aspergillus brasiliensis ATCC® 16404 was not visible with competitor products (Table 4).
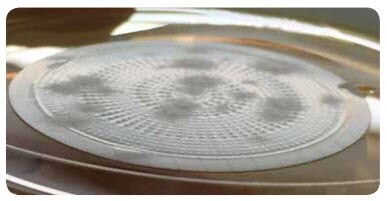
Figure 5.Example of visible growth of Aspergillus brasiliensis ATCC® 16404 after a 48 hour incubation
Conclusion
This study demonstrates that EZ-Fit® Filtration Units exhibit a good recovery rate with both SDA and TSA in all filtration rounds. A constant detection of Aspergillus brasiliensis ATCC® 16404 is possible.
The easy-to-use handling of EZ-Fit® Filtration Units offers positive advantages for daily lab work, and supports constant results with innovative design and highly qualitative used materials.
There are several factors that influence microbial recovery by membrane filtration. All of the products tested here have cellulose based membranes or cellulose nitrate membranes (Table 1). Mixed cellulose ester (MCE) membranes may differ in the ratio to cellulose nitrate, and this can affect recovery. Sterilization procedures must also be designed and controlled to maintain optimal recovery.
Thus the membrane composition, raw material sources and sterilization processes may all help to explain why in this study the EZ-Fit® Filtration Units offers better recovery than competitive membrane filters.
Further Reading and Information
1. European Pharmacopoeia Chapter 2.6.12: Microbiological examination of non-sterile products: Total viable aerobic count
2. U.S. Pharmacopeia <1231> Water for Pharmaceutical Purposes
3. Japanese Pharmacopeia 16th Edition JP Chapter 4.4.2
4. U.S. Pharmacopeia <1227> Validation of Microbial Recovery
5. ISO International Standardization Organization: Water quality – Evaluation of membrane filters
如要继续阅读,请登录或创建帐户。
暂无帐户?