Cytochrome C in Molecular Evolution
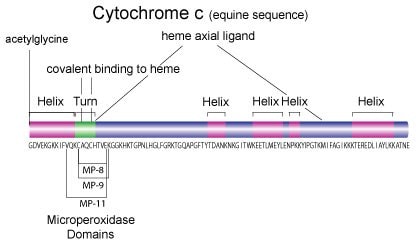
Cytochrome c is a highly conserved ~12 kDa protein consisting of a single 104 amino acid peptide with a single heme group, which is covalently attached to Cys14 and Cys17. Because of its ubiquitous nature and sequence homology, cytochrome c has been used as a model protein for molecular evolution.1
On this page:
Sequence Homology


Molecular mass:3
12,384 Da (equine)
12,327 Da (bovine)
12,384 Da (pigeon)
12,588 Da (Saccharomyces cerevisiae)
12,233 Da (human)
Isoelectric point (pI):4 range of 10.0 – 10.5 (equine)
Spectral properties:5 (equine)
λmax = 550 nm (reduced form)
EmM = 29.5 (reduced form, 0.1 M phosphate buffer, pH 6.8)
EmM = 8.4 (oxidized form, 0.1 M phosphate buffer, pH 6.8)
Recommended storage time for aqueous solutions
Storage at –20 °C (freezer): 6 months
Storage at 2-8 °C (refrigerator): 2 weeks
Storage at 20-25 °C (ambient temperature): 3 days
Electron Transport
Cytochrome c is primarily known as an electron-carrying mitochondrial protein. The transition of cytochrome c between the ferrous and ferric states within the cell, makes it an efficient biological electron-transporter and it plays a vital role in cellular oxidations in both plants and animals. It is generally regarded as a universal catalyst of respiration, forming an essential electron-bridge between the respirable substrates and oxygen. Its main function in cellular respiration is to transport electrons from cytochrome c reductase (Complex III) to cytochrome oxidase (Complex IV).
Apoptosis
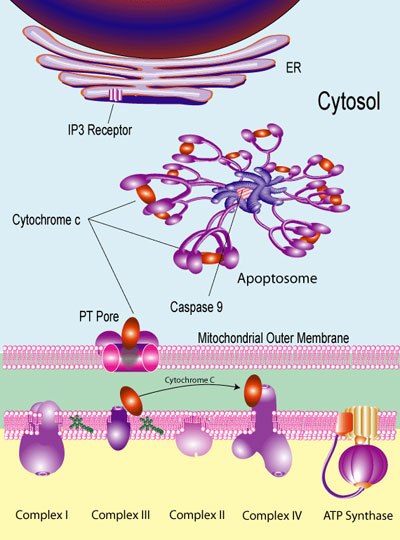
More recently, cytochrome c has been identified as an important mediator in apoptotic pathways.5 The release of mitochondrial cytochrome c into the cytoplasm stimulates apoptosis and is commonly used as an indicator of the apoptotic process in the cell.6 Serum cytochrome c levels may be an indicator of therapy-induced cell death burden7
Under proapoptotic conditions, two Bcl-2 family proteins, Bax and Bak associate with the voltage-dependent anion channel component of the permeability transition (PT) pores on the outer membrane of the mitochondria. This calcium-dependent process allows the release of cytochrome c from the intermembrane space of the mitochondria into the cytoplasm.6
The initial release of cytochrome c into the cytoplasm can result in the association of cytochrome c with the inositol-3-phosphate receptor (IP3 receptor) which acts as a calcium channel on the outer membrane of the endoplasmic reticulum. Subsequent release of calcium ions into the cytoplasm can induce apoptosis.5
Cytochrome c also participates in the cytosolic caspase proteolytic cascade of apoptosis as a component of the apoptotic protease activating factor (Apaf). The association of cytochrome c with Apaf-1 results in the formation of the apoptosome protein complex which can recruit and activate pro-caspase 9 (Apaf-3). Activation of caspase 9 then facilitates the downstream activation of caspases 3 and 7 resulting in apoptosis.8 The cytochrome c-mediated release of calcium from the ER helps to initiate the apoptotic cascade since activation of both caspase 9 and caspase 3 is calcium-dependent.9
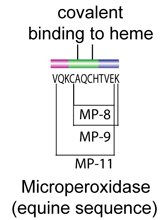
Cytochrome c has been shown to exhibit peroxidase activity. This activity is preserved in small peptide fragments that retain the bound heme group. These heme-containing peptides are termed microperoxidase and have found utility in histological absorption studies.
T-Cell Receptor Studies
The unique sequence of pigeon cytochrome c is utilized for activation of specific T-cell hybridoma receptors.10
Use as a Protein Standard
Cytochrome c is commonly used as a control protein for several applications:
- Molecular weight standard for gel filtration chromatography
- Mass spectrometry MW calibrant
- Peptide map control
- Solid phase protein sequencing analysis standard
- Universal proteomics standard (cytochrome c component)
Methods of Preparation
11 Alternatively, acetic acid may be used to prepare cytochrome c. The trichloroacetic acid method may reduce the amount of superoxide dismutase (SOD) present, but tends to cause dimerization or acid-modified structures of cytochrome c. In contrast, acetic acid preparations may have slightly higher amounts of SOD, but a lower proportion of dimeric cytochrome c.
OXIDATIVE STATES OF CYTOCHROME c
We supply cytochrome c products predominantly in the oxidized form of the protein. The reduced form of cytochrome c can be prepared with either sodium dithionite or sodium ascorbate, followed by gel filtration.12
The following procedure is taken from our assay procedure for cytochrome oxidase. Dissolve 100 mg of cytochrome c in 8 mL of 10 mM potassium phosphate buffer, pH 7.0. Reduce the cytochrome c by adding 3–5 mg of L-ascorbic acid, sodium salt (A7631). The excess ascorbate is removed by dialyzing against 10 mM potassium phosphate buffer, pH 7.0, for 18–20 hours at 0–4 °C with three changes of buffer. Remove from dialysis and bring up to a final volume of 10 mL with the buffer.
Cytochrome c Products
- Cytochrome c Proteins
- Cytochrome c Control Proteins
- Related Kits and Reagents
- Microperoxidase
- BioUltra Cytochrome c from Equine Heart

BioUltra Cytochrome c ≥99% (SDS-PAGE) from Equine Heart We offer a BioUltra grade cytochrome c for applications where the highest purity available is required. |
Catalog Number: C2867
Synonym: Ferricytochrome c (oxidized state)
This cytochrome c product is prepared from equine heart using trichloroacetic acid by a modification of a published method.11 Alternatively, acetic acid may be used to prepare cytochrome c (C7752). The trichloroacetic acid method may reduce the amount of superoxide dismutase (SOD) present, but tends to cause dimerization or acid-modified structures of cytochrome c. In contrast, acetic acid preparations may have slightly higher amounts of SOD, but a lower proportion of dimeric cytochrome c.
The product is supplied as a lyophilized powder. The final step before lyophilization is extensive dialysis against 6 mM ammonium hydroxide, which is volatile under lyophilization conditions, so the final product should not contain any buffer salts. The product is mainly the oxidized form of the protein. The reduced form of cytochrome c can be prepared with either sodium dithionite or sodium ascorbate, followed by gel filtration.12
Analysis of Representative Lots
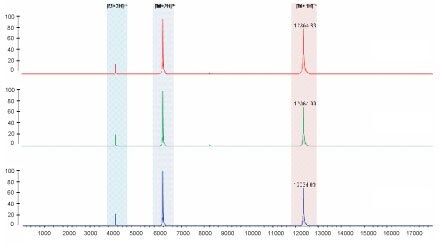
MALDI-ToF mass spectrometry analysis of three representative lots of C2867 Cytochrome c.
Results indicate only the three charge states of cytochrome c are detected.

Purity: 99% by SDS-PAGE
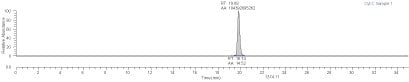
Purity: 95% by spectral assay
Purity confirmed on representative lots by HPLC-electrospray MS. HPLC analysis (C2867) using TFA:ACN gradient on Supelco Discovery® 15 cm x 2.1 mm C8, UV detection at 214 nm.
Preparation Instructions
Cytochrome c is soluble in water or buffered solutions at pH 7.0±1.0 (up to 200 mg/mL), yielding a clear, dark red solution. For general purposes reconstitution is done at 10 mg/mL.
Storage/Stability
Store cytochrome c at –20 °C. The product, as supplied, is stable for 5 years.
Recommended storage time for aqueous solutions
Storage at –20 °C (freezer): 6 months
Storage at 2-8 °C (refrigerator): 2 weeks
Storage at 20-25 °C (ambient temperature): 3 days
References
如要继续阅读,请登录或创建帐户。
暂无帐户?