Gene Therapy Manufacturing
The promise of new gene therapies for patients with few or no treatments available is driving demand for rapid innovation, with hundreds of gene therapies now in development worldwide. But bringing a new gene therapy to life demands a high degree of expertise in viral vector manufacturing and testing. Yet there are many challenges to producing a gene therapy including accelerated time-to-patient, lack of an established process template, and evolving regulatory guidelines.
Gene therapy manufacturers must find their own path through complex obstacles in process development, scale-up, manufacturing, and regulatory compliance – all within compressed timelines of just 3-5 years, less than half the time for conventional drugs.
Successful manufacturers must also show agility in clearing regulatory, safety, and process development hurdles. Consequently, alliances with experienced global technology partners or contract development and manufacturing organizations (CDMOs) are often essential for bringing a gene therapy to life.
Related Product Resources
Article: Anticipating the Next Decade of Gene Therapy
Article: Beyond 2020: Looking into the Crystal Ball for Gene Therapy
Infographic: Making Rare Disease Therapies Less Rare (Labiotech)
Article: Regionalizing and Democratizing Cell and Gene Therapy
Article: Perspectives of Value-driven, Integrated Solutions in Gene Therapy
Gene therapy development: Grow, purify, formulate
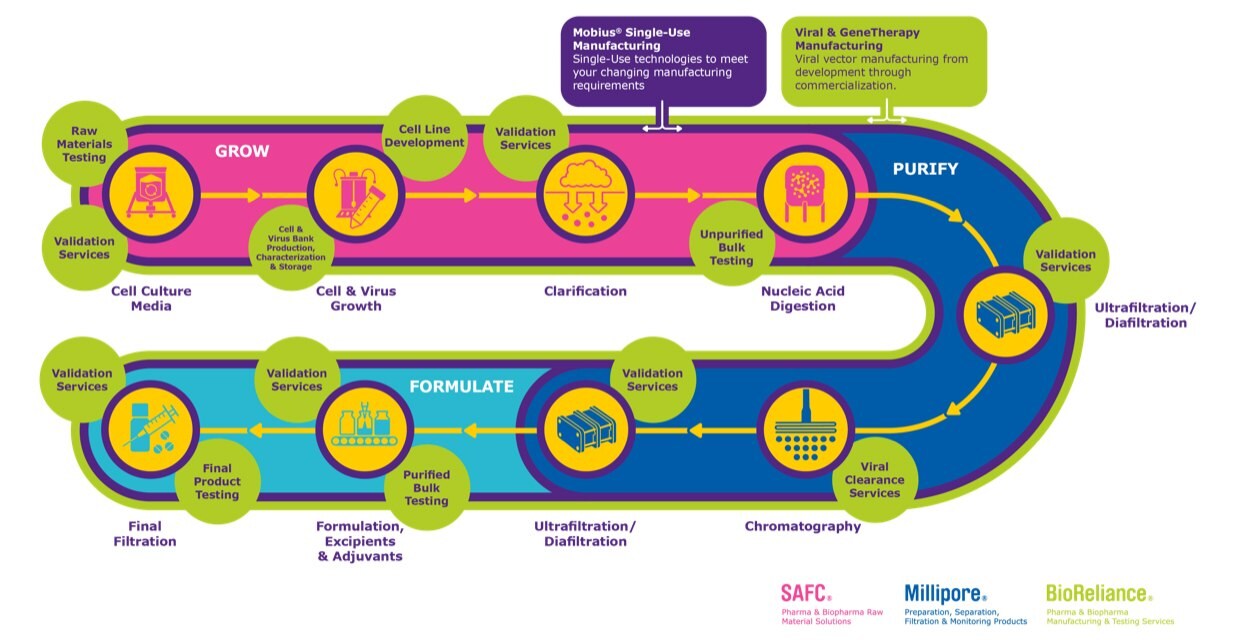
Workflow
Viral Vector Downstream Processing
Efficient virus purification processes can improve yield, decrease time to patient, and lower manufacturing costs
Viral Vector Formulation and Final Fill
Formulating a commercially viable gene therapy demands a high level of application and regulatory expertise
Viral Vector Characterization and Biosafety Testing
Critical biosafety testing and characterization of viral vector products can help to fully analyze key quality attributes: identity, potency, safety, and stability
Viral Vector Contract Development and Manufacturing
CDMO partnerships play a critical role in advancing clinical pipelines and achieving successful commercialization
To continue reading please sign in or create an account.
Don't Have An Account?