Cell Culture Protocol 4: Subculture of Semi-Adherent Cell Lines
ECACC Laboratory Handbook 4th Edition
Aim
Some cell lines grow as a mixed population (e.g. B95-8 - marmoset) where a proportion of cells do not attach to the tissue culture flask and remain in suspension. Therefore, to maintain this heterogeneity both the attached cells and the cells in suspension must be subcultured.
Materials
- Cell Culture Media: pre-warmed to the appropriate temperature (refer to the ECACC cell line data sheet for the correct medium and temperature.)
- 70% (v/v) alcohol in sterile water (793213)
- PBS without Ca2+/Mg2+ (D8537)
- 0.05% trypsin/EDTA in HBSS, without Ca2+/Mg2+ (T3924)
- Soybean Trypsin Inhibitor (T6522) or FBS
- Trypan Blue Solution (T8154)
Equipment
- Personal protective equipment (sterile gloves, laboratory coat, safety visor)
- Waterbath set to appropriate temperature
- Microbiological safety cabinet at appropriate containment level
- Incubator
- Pre-labelled flasks
- Inverted phase contrast microscope
- Centrifuge
- Hemocytometer or automated cell counter like a Scepter™ Cell Counter
- Marker Pen
- Pipettes
- Ampoule rack
- Tissue
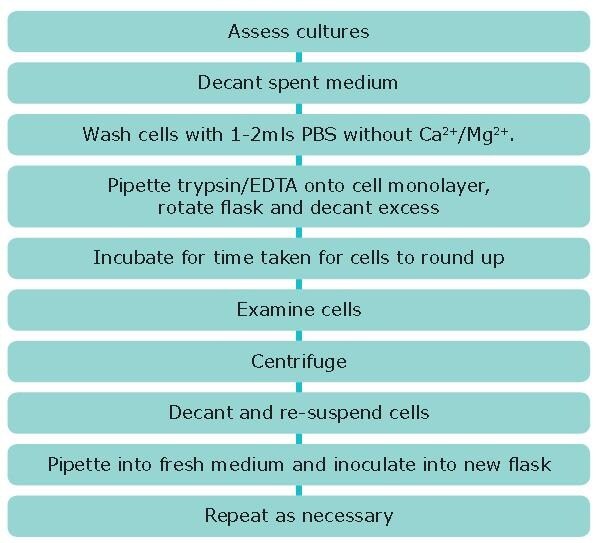
Procedure

- View cultures using an inverted phase contrast microscope to assess the degree of confluency and confirm the absence of bacterial and fungal contaminants. Give the flask a gentle knock first, this may dislodge the cells from the flask and remove the need for a trypsinization step loss of some cells due to associated washes.
- Decant medium containing non-adherent cells into a sterile centrifuge tube and retain.
- Wash any remaining attached cells with PBS without Ca2+/Mg2+ using 1-2 mL for each 25 cm2 of surface area. Retain the washings.
- Pipette trypsin/EDTA onto the washed cell monolayer using 1 mL per 25cm2 of surface area. Rotate flask to cover the monolayer with trypsin. Decant the excess trypsin.
- Return flask to incubator and leave for 2-10 minutes.
- Examine the cells using an inverted microscope to ensure that all the cells are detached and floating. The side of the flasks may be gently tapped to release any remaining attached cells.
- Transfer the cells into the centrifuge tube containing the retained spent medium and cells.
- Centrifuge the entire cell suspension at 150 x g for 5 minutes.
- Remove the supernatant and re-suspend the cell pellet in a small volume (10-20 mL) of fresh culture medium. Count the cells.
- Pipette the required number of cells into a new labelled flask and dilute to the required volume using fresh medium (refer to ECACC Cell Line Data Sheet for the required seeding density).
- Repeat this process every 2-3 days as necessary.
Key Points
- Although most cells will detach in the presence of trypsin alone the inclusion of EDTA enhances the activity of the enzyme by removing inhibitory cations.
- Trypsin is inactivated in the presence of serum. Therefore, it is essential to remove all traces of serum from the culture medium by washing the monolayer of cells with PBS without Ca2+/Mg2+. Repeated warming to 37 °C also inactivates trypsin.
- Cells should only be exposed to trypsin/EDTA long enough to detach cells. Prolonged exposure could damage cell surface receptors. In general, a shorter time of exposure to trypsin is required for semi-adherent cell lines in comparison to adherent cell lines.
- Trypsin should be neutralized with serum prior to seeding cells into new flasks otherwise cells will not attach.
- Trypsin may also be neutralized by the addition of Soybean Trypsin Inhibitor, where an equal volume of inhibitor at a concentration of 1mg/mL is added to the trypsinized cells. The cells are then centrifuged, resuspended in fresh culture medium and counted as above.
- If a CO2 incubator is not available gas the flasks for 1-2 minutes with 5% CO2 in 95% air filtered through a 0.2μm filter.
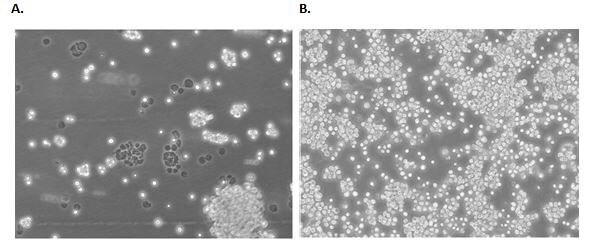
Figure 1. Examples of a semi-adherent cell line.B95-8 cells 24 hours (A) and 72 hours (B) post freeze/thaw show semi-adherent fibroblastic and lymphoblastic morphology. Confluence should carefully be monitored and passaged when cells reach ~80% confluency.
Sign In To Continue
To continue reading please sign in or create an account.
Don't Have An Account?