SnAP Reagents
Introduction to Saturated N-heterocycle Building Blocks (SnAP)
Saturated N-heterocyclic building blocks are of growing importance but their installation has been challenging due to poor commercial availability and the often long, laborious synthetic routes needed to form these ring systems.
SnAP Reagents are an ever-expanding class of reagents, which are now available for the convenient synthesis of medium-ring (6-9 membered) saturated N-heterocycles, including bicyclic and spirocyclic structures. In collaboration with the Bode research group, many of the SnAP Reagents are now commercially available, and custom-made reagents for specific applications or targets can also be prepared from simple starting materials.
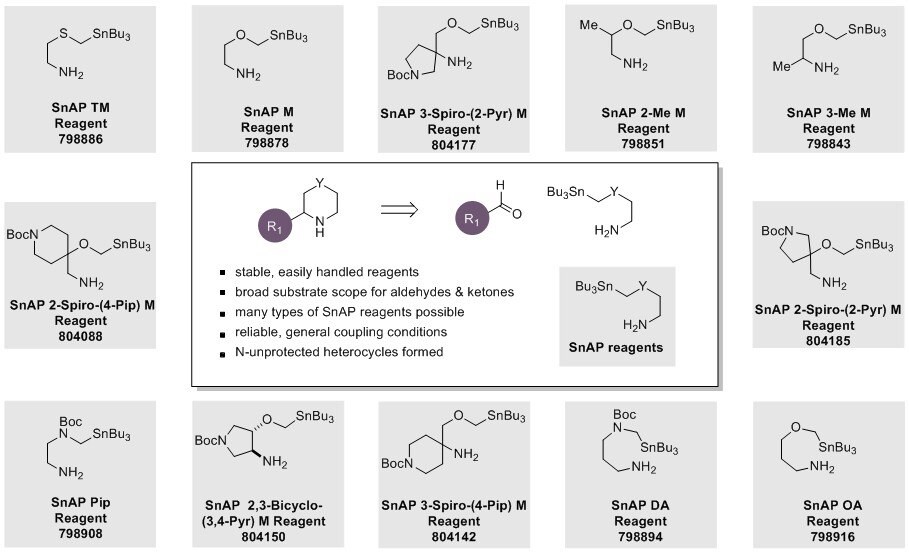
Advantages of SnAP Reagents
SnAP reagents are stable and readily available and can be coupled with widely available aromatic, heteroaromatic, aliphatic, and glyoxylic aldehydes using versatile and predictable methodology, developed within the Bode group, for the synthesis of saturated N-heterocycles. A general procedure from the review is shown below.
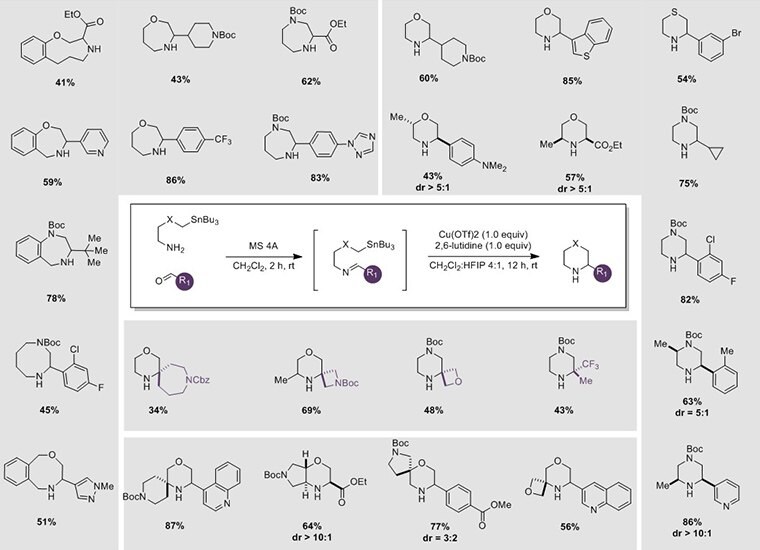
Procedure for Synthesis of N-Heterocycles Using SnAP Reagents
- 1 equiv (0.50 mmol) of amino tributylstannane (SnAP Reagent) was dissolved in 2.5 mL CH2Cl2.
- To this solution, 1 equiv (0.50 mmol) of the correspond aldehyde and 4Å molecular sieves (ca. 100 mg/mmol) were added. The reaction mixture was stirred at room temperature for 2 hours and filtered through a short layer of Celite (CH2Cl2 rinse).
- The filtrate was concentrated under reduced pressure to afford the pure imine.
- Separately, 1 equiv (0.50 mmol) of 2,6-lutidine, 2.0 mL of HFIP, and 1 equiv (0.50 mmol) of anhydrous Cu(OTf)2 were stirred at room temperature for 1 hour, forming a homogeneous suspension.
- To this homogeneous suspension, 8.0 mL CH2Cl2 and the imine from Step 3 was added at once and stirred at room temperature for 12 hours.
- The reaction was quenched with 5 mL 10% aq NH4OH and stirred vigorously for 15 minutes.
- The layers were separated and the aqueous layer was extracted with CH2Cl2 (3 x 3 mL). The combined organic layers were washed with H2O (3 x 5 mL) and brine (10 mL), dried over Na2SO4, filtered, and concentrated.
- Purification by flash column chromatography affords the corresponding N-heterocycle.
References
To continue reading please sign in or create an account.
Don't Have An Account?