Certified Reference Materials: Synthesis and Characterization of Cocaine N-oxide HCl
Yunming Ying, Brian Schulmeier, Kenan Yaser, Uma Sreenivasan, Kelley Huff, Beth Marek, Isil Dilek
Cerilliant Corporation, 811 Paloma Drive, Suite A, Round Rock, TX 78665
Introduction
Cocaine is one of the most widespread illicit drugs of abuse in the US. Cocaine N-oxide is a metabolite of cocaine formed by cytochrome P-450 mediated oxidation of cocaine.1,2 Cocaine N-oxide has been reported to be found in human plasma of cocaine users as well as meconium.1 Cocaine N-oxide isomers have been observed in human hair samples in varying amounts (personal communication, Dr. Mark Miller, FBI Quantico). Development of accurate methods to detect and quantitate cocaine N-oxide has applications in forensic / toxicology applications and in the screening for newborns when cocaine use in the parent is suspected.
Cocaine N-oxide is heat sensitive and readily degrades to cocaine and norcocaine on column when analyzed by GC/MS.1,2 Testing laboratories are developing LC/MS methods for this metabolite to monitor cocaine use in matrices such as hair. This poster presents the synthesis and stability of Certified Reference Materials (CRMs) of high purity cocaine N-oxide and its internal standard.
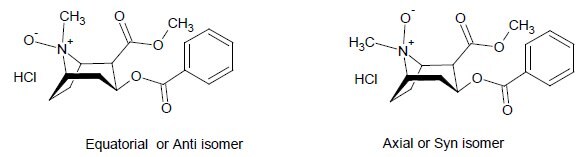
Cocaine N-oxide is a mixture of equatorial (anti) and axial (syn) isomers based on orientation of the N-oxide relative to substituents on the tropine ring.3
References
Synthesis of Cocaine N-oxide HCl
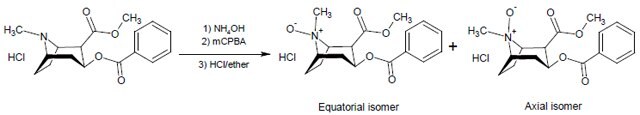
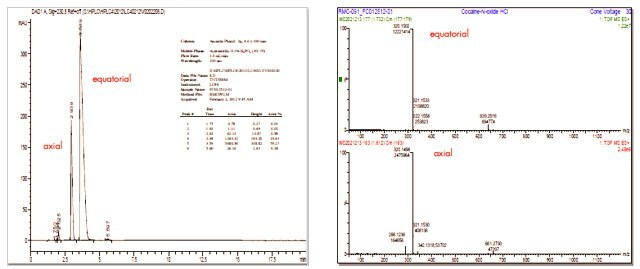
Cocaine N-oxide HCl was synthesized from cocaine HCl by oxidation with mCPBA in chloroform at 0 °C. Initial attempts to isolate pure cocaine N-oxide failed as the free base is unstable. Cocaine N-oxide was then converted to its hydrochloride salt in situ and purified to >98% (by HPLC), with an approximately 79/19 mixture of equatorial and axial isomers.
HPLC and LC/MS of Cocaine N-oxide HCl (Mixture of Equatorial and Axial Isomers)
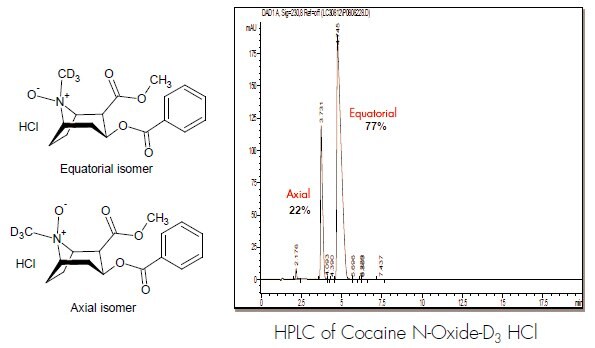
Internal Standard: Cocaine N-oxide-D3 HCl
Cocaine N-oxide-D3 HCl was synthesized for use as an internal standard in a similar manner from cocaine-D3 HCl.
Identification of the Equatorial and Axial Isomers of Cocaine N-oxide HCl By NMR
1H and 2D 1H-1H-COSY NMR were used to identify the two isomers.
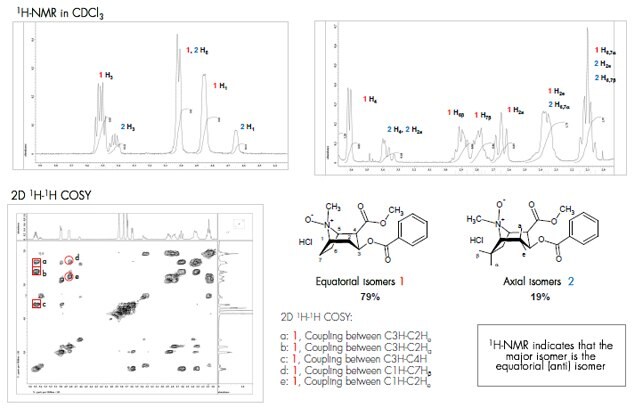
The two isomers of cocaine N-oxide HCl were not stable enough to be individually isolated. 1H-NMR and 2D 1H-1H COSY spectra were obtained for the mixture of the isomers. The NMR spectra were measured in CDCl3 on a JEOL ECS-400 spectrometer. The1H-NMR shows two distinct sets of signals ~ 80:20, similar to the HPLC.
- Two broad non-resolved signals at 4.85 and 4.65 ppm can be assigned to the bridgehead proton at C1.
- The torsion angle between the N-oxide bond and C1-H bond in the equatorial isomer1 is smaller than in the axial isomer 2. Therefore, the bridgehead proton at C1 in 1 is more deshielded and is represented by the signal at 4.85 ppm (80%), while the signal at 4.65 ppm (20%) represents the axial isomer 2.
- The two pentet signals at 5.52 ppm (1) and 5.42 ppm (2) represent the protons at the C3 position, with a slight deshielding effect for 1.
- Other proton signals were assigned by 2D 1H-1H COSY NMR and comparison with the isomers of tropine N-oxide.3
Cocaine N-oxide HCl Certified Reference Material: Development and Stability
Cocaine N-oxide HCl and the internal standard were developed into ampouled solution-based Certified Reference Materials for use in identification and quantitation of cocaine use. The standard solution of cocaine N-oxide HCl was prepared at 1.0 mg/mL in acetonitrile. The internal standard was prepared at 100 μg/mL. Accelerated and real-time stability were performed for both native and labeled standards.
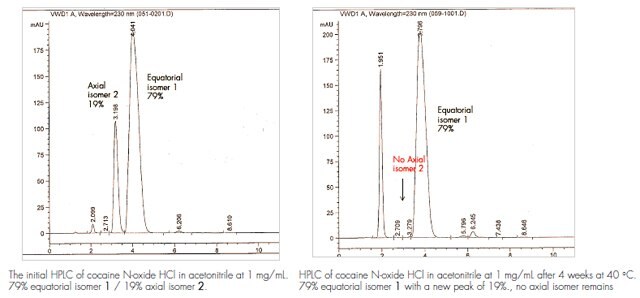
Cocaine N-oxide HCl Certified Reference Material: Development and Stability
Accelerated and real-time stability of cocaine N-oxide HCl evaluated by HPLC is shown in Table 1
- Degradation is observed at refrigerator, room temperature and 40 °C storage conditions.
- After 4 weeks at 40 °C, no axial isomer was left, while the equatorial isomer stayed at 79%. The major impurity is 19% with m/z 306 by LC/MS.
Cocaine N-oxide HCl CRM is stable under sub-freezer and freezer storage conditions for 1 year
Stability data for cocaine N-oxide-D3 HCl at 100 μg/mL in acetonitrile is shown in Table 2
- Cocaine N-oxide-D3 HCl is stable under sub-freezer condition.
- The degradation rate in the freezer, refrigerator, room temperature and 40 °C was higher than in the 1 mg/mL native solution.
- Similar to its native counterpart, no axial isomer was left after 4 weeks at 40 °C while the equatorial isomer remained at 79% by HPLC. The major impurity has m/z 309 by LC/MS, consistent with native.
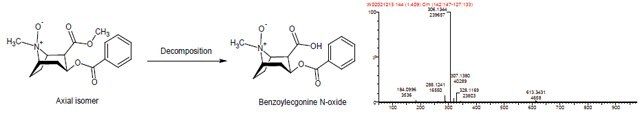
Decomposition of the Axial Isomer
- The decomposition product of the axial isomer of cocaine N-oxide HCl has m/z of 306 corresponding to either norcocaine N-oxide or benzoylecgonine N-oxide.
- The decomposition product is proposed to be benzoylecgonine N-oxide based on the mechanism of decomposition: in the axial isomer, the N-oxide can facilitate hydrolysis of the methyl ester through intramolecular assistance from the syn (axial) N-oxide.
- Faster degradation observed for lower concentration labeled standard maybe due to the higher dilution.
Conclusion
- Cocaine N-oxide HCl was synthesized and purified to >98% purity as a mixture of equatorial (anti) and axial (syn) isomers. The equatorial isomer is predominant.
- Cocaine N-oxide decomposes in solution, with loss of a methyl group. Metabolically, cocaine N-oxide is reported to be converted to norcocaine by P-450 mediated oxidtation.4 In solution, the decomposition product is proposed to be benzoylecgonine N-oxide. The axial (syn) isomer is the major contributor to the decomposition. Degradation is most likely facilitated by intramolecular contribution from the axial N-oxide.
- The variability of the isomers observed in human samples may be due to the rapid degradation of the axial isomer. In the analysis of Cocaine N-oxide in biological matrices, quantitation and detection may vary depending on ratio of axial and equatorial isomers in the sample, and the extent of degradation of the axial isomer that occurs, in solution, during sample preparation and analysis. Trace amounts of the m/z 306 degradation product may provide information on presence of the axial isomer in the sample.
- Cocaine N-oxide HCl and the D3 internal standard were developed as solution-based Certified Reference Materials for screening and quantitative applications.
- Cocaine N-oxide HCl CRM at 1 mg/mL in acetonitrile is stable under freezer and sub-freezer conditions >1 year, but decomposes within days at room temperature and 40 °C. Six months of real-time stability is available for the internal standard with additional work in progress.
To continue reading please sign in or create an account.
Don't Have An Account?