Astec Cellulose DMP
Efficient, Rugged, and Economical Columns for Chiral HPLC & SFC
Astec Cellulose DMP is a chiral stationary phase (CSP) comprising spherical, high-purity porous silica coated with DMPC (3,5-dimethylphenyl carbamate)-derivatized cellulose, and packed in analytical to preparative size HPLC columns. It separates a wide range of chiral compounds under normal phase, polar organic, and SFC conditions, with high efficiency, high loading capacity, and excellent column lifetime. Performance is comparable to other DMPC-derivatized cellulose CSPs.
Key Features and Application Areas:
- Classic DMPC-cellulose chiral selectivity
- Efficient, rugged, reproducible, and scalable
- Low backpressure
- Ideal for chiral analysis in the pharmaceutical industry and for small analytes in chemical and environmental areas
- Routine chiral column method development screening protocols
- Approximately one-half the cost of most DMPC-cellulose columns
Astec Cellulose DMP is complementary to the other Astec CSPs, including CHIROBIOTIC®, CYCLOBOND™, and the P-CAP™ product lines, and a must-have for every chiral HPLC or SFC screening protocol.
Contents:
- What is Cellulose?
- How do Cellulose-Based CSPs Separate Enantiomers?
- What Makes Astec Cellulose DMP Unique?
- Normal Phase Chiral Separations
- Comparability to Other DMPC-Cellulose Columns
- Normal Phase Applications
- Polar Organic Mode (POM) Chiral Separations
- SFC Chiral Separations
- Ideal for Preparative Applications
- Complementary to the Other Astec CSPs
What is Cellulose?
The polysaccharide cellulose is a naturally occurring, optically active, linear polymer comprising hundreds to thousands of D-(+)-glucose units joined by ß1,4-glycosidic bonds. The long polysaccharide chains form rope-like bundles held together via multiple hydrogen bonds between proximate hydroxyl groups. In 1973, Hesse and Hagel described the enantioselective properties of microcrystalline cellulose triacetate.1 In the mid-1980’s, Okamoto and colleagues published their work that lead to the use of derivatized cellulose adsorbed onto silica as chiral HPLC stationary phases.2,3 Since then, the polysaccharides, particularly cellulose and amylose, have become the most commercially successful class of CSPs.
How do Cellulose-Based CSPs Separate Enantiomers?
Derivatized cellulose-based CSPs, like Astec Cellulose DMP, owe their high enantioselectivity to the large number of chiral centers in the polysaccharide backbone and to its highly-ordered structure. The shape of the pockets formed by the intertwined chains provides chiral discrimination based on molecular shape.
Derivatives at the 2, 3, and 6-position hydroxyls confer additional enantioselectivity. The dimethylphenyl carbamate derivative (Figure 1) separates a wide range of enantiomers; the phenyl ring and carbamate groups provide p-p and hydrogen bonding interactions, respectively, with predisposed analytes.
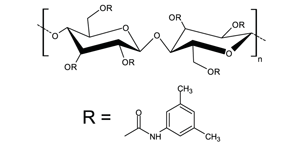
Figure 1. DMPC-Cellulose Structure- Cellulose is a linear polymer of D-(+)-glucose units linked by β1,4-glycosidic bonds. This figure shows the cellulose tris(3,5-dimethylphenylcarbamate) derivative used in Astec Cellulose DMP.
What Makes Astec Cellulose DMP Unique?
Astec Cellulose DMP is unique in offering classic DMPC-cellulose chiral selectivity, but at approximately one-half the cost of most DMPC-cellulose columns. It is an ideal component of any chiral column screening portfolio, and should be investigated as an alternative to higher-priced DMPC-cellulose columns for existing methods. The cost savings are especially dramatic when comparing preparative column dimensions.
Normal Phase Chiral Separations
DMPC-derivatized cellulose is commonly run in normal phase mode. Typical normal phase mobile phases are hexane or heptane with ethanol or IPA as polar modifiers. The performance of Astec Cellulose DMP in terms of selectivity and compatibility under normal phase conditions meets or exceeds competitive phases of similar composition. Figures 2-4 show the resolution of three racemates on Astec Cellulose DMPC and two higher-priced competitive phases.
Comparable to Other DMPC-Cellulose Columns
For the compounds we have tested, Astec Cellulose DMP columns provide similar retention and selectivity, but lower backpressure and higher efficiency compared to competitive columns. Astec Cellulose DMP is not a clone of other DMPC-derivatized cellulose columns on the market, but selectivity and retention is similar enough to use it instead of these columns in your chiral column HPLC and SFC screening protocols. It also should be investigated as a possible replacement for these columns in established methods. The data in Table 1 compares resolution, pressure, and selectivity on Astec Cellulose DMP and the leading competitive column. Comparison also appears in Figures 2-4 against two competitive columns.
Figure 2. Tröger’s Base - Competitive Comparison
columns: 15 cm x 4.6 mm I.D., 5 μm particles
mobile phase: 10:90:0.1, IPA:heptane:DEA
flow rate: 0.5 mL/min.
temp.: 25 °C
det.: UV, 230 nm
inj.: 2 μL
sample: Tröger's Base (2 mg/mL)
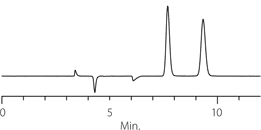
Astec Cellulose DMP
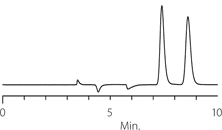
Competitor D
Competitor P
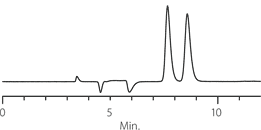
Figure 3. Metoprolol - Competitive Comparison
columns: 15 cm x 4.6 mm I.D., 5 μm particles
mobile phase: 10:90:0.1, IPA:heptane:DEA
flow rate: 0.5 mL/min.
temp.: 25 °C
det.: UV, 230 nm
inj.: 2 μL
sample: metoprolol
Astec Cellulose DMP
Competitor D
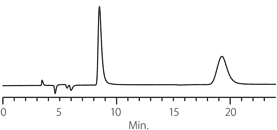
Competitor P
Figure 4. Diperodon - Competitive Comparison
columns: 15 cm x 4.6 mm I.D., 5 µm particles
mobile phase: 0.1% ammonium formate in methanol
flow rate: 0.5 mL/min.
temp.: 25 °C
det.: UV, 230 nm
inj.: 2 µL
sample: diperodon (2 mg/mL)
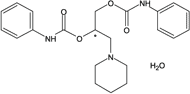
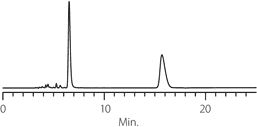
Astec Cellulose DMP
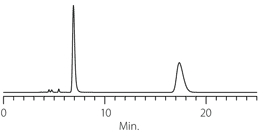
Competitor D
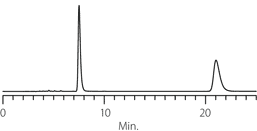
Competitor P
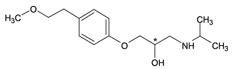
Figure 5. Alprenolol
column: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 µm particles (51098AST)
mobile phase: 10:90:0.1, IPA:heptane:DEA
flow rate: 0.5 mL/min.
temp.: 25 °C
det.: UV, 254 nm
inj.: 2 µL
sample: alprenolol (2 mg/mL in mobile phase)
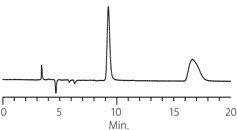
Figure 6. Atropine
column: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 µM particles (51098AST)
mobile phase: 10:90:0.1, IPA:heptane:DEA
flow rate: 0.5 mL/min.
temp.: 25 °C
det.: UV, 254 nm
inj.: 2 µL
sample: atropine (2 mg/mL in mobile phase)
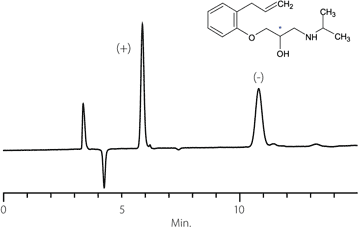
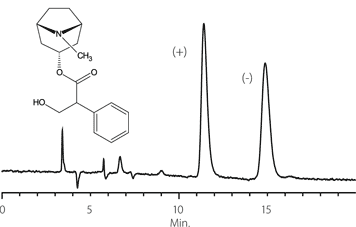
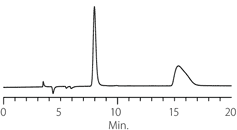
Figure 7. Benzoin
column: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 μm particles (51098AST)
mobile phase: 10:90:0.1, IPA:heptane:TFA
flow rate: 0.5 mL/min.
temp.: 25 °C
det.: UV, 254 nm
inj. 2 μL
sample: benzoin (2 mg/mL in mobile phase)
Figure 8. Hydroxyzine
column: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 μm particles (51098AST)
mobile phase: 10:90:0.1, IPA:heptane:DEA
flow rate: 0.5 mL/min.
temp.: 25 °C
det.: UV, 254 nm
inj.: 2 μL
sample: hydroxyzine (2 mg/mL in mobile phase)
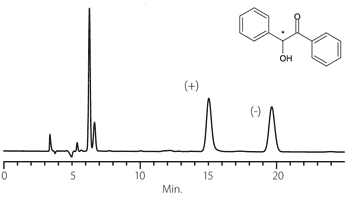
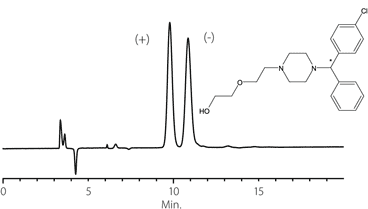
Figure 9. Proglumide
column: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 μm particles (51098AST)
mobile phase: 10:90:0.1, IPA:heptane:TFA
flow rate: 0.5 mL/min.
temp.: 25 °C
det.: UV, 254 nm
inj.: 2 μL
sample: proglumide (2 mg/mL in mobile phase)
Figure 10. Etodolac
column: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 μm particles (51098AST)
mobile phase: 10:90:0.1, IPA:heptane:TFA
flow rate: 0.5 mL/min.
temp.: 25 °C
det.: UV, 254 nm
inj.: 2 μL
sample: etodolac (2 mg/mL in mobile phase)
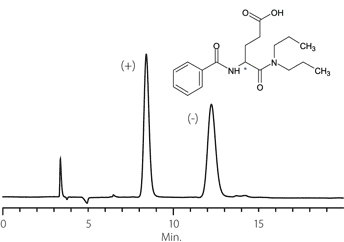
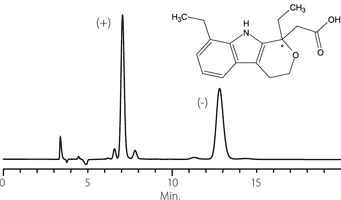
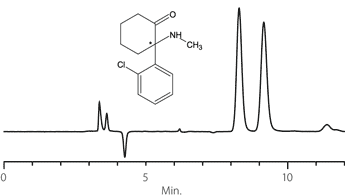
Figure 11. Ketamine
column: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 μm particles (51098AST)
mobile phase: 10:90:0.1, IPA:heptane:DEA
flow rate: 0.5 mL/min.
temp.: 25 °C
det.: UV, 254 nm
inj.: 2 μL
sample: ketamine (2 mg/mL in mobile phase)
Figure 12. Warfarin
column: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 μm particles (51098AST)
mobile phase: 100:0.2:0.1, CH3OH:acetic acid:TEA
flow rate: 0.5 mL/min.
temp.: 25 °C
det.: UV, 278 nm
inj.: 2 μL
sample: warfarin (2 mg/mL in mobile phase)
Polar Organic Mode (POM) Chiral Separations
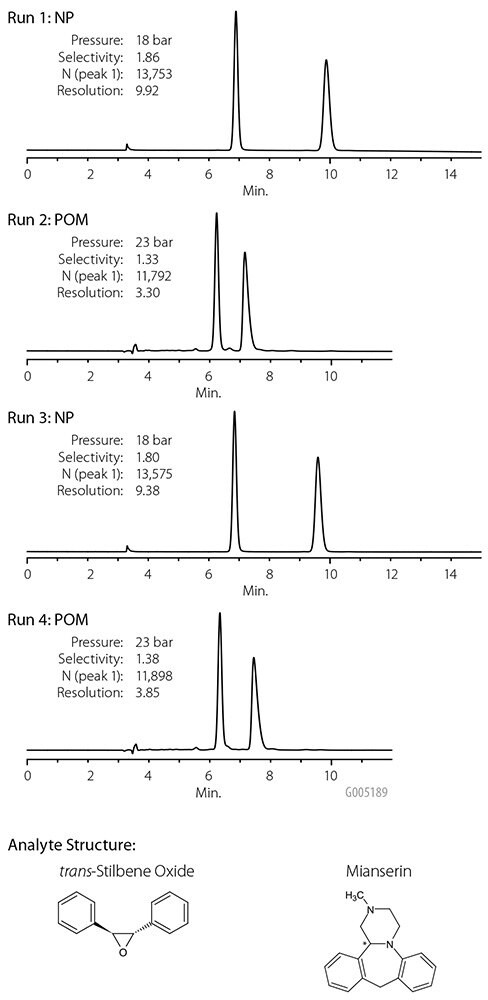
Polar organic mode (POM) mobile phases comprise acetonitrile and/or methanol, often with acids, bases, or salts added to control peak shape and retention of certain sensitive analytes. The benefit of POM is realized when dealing with compounds that are poorly soluble in non-polar normal phase mobile phases. For preparative chiral applications, solubility is especially important; analyte concentration per injection influences the throughput. Astec Cellulose DMP operates in POM to permit choice of mobile phase based on analyte solubility. A few of the racemates resolved on Astec Cellulose DMP with different POM mobile phases appear in Table 2 and Figures 13-15. Not only does Astec Cellulose DMP provide excellent resolution in POM and NP modes, it also is rugged enough to hold up to repeated NP-POM-NP-POM cycles without loss of performance. Figure 16 demonstrates the same Astec Cellulose DMP column used alternately in normal phase and POM mobile phases. Performance values appear in the figure.
Figure 13. Homatropine
column: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 μm particles (51098AST)
mobile phase: 0.1% v/v DEA in methanol
flow rate: 0.5 mL/min.
temp.: 25 °C
det.: UV, 230 nm
inj.: 2 μL
sample: homatropine (2 mg/mL in mobile phase)
Figure 14. Indapamide
column: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 μm particles (51098AST)
mobile phase: 0.1% w/v ammonium formate in methanol
flow rate: 0.5 mL/min.
temp.: 25 °C
det.: UV, 230 nm
inj.: 2 μL
sample: indapamide (2 mg/mL in mobile phase)
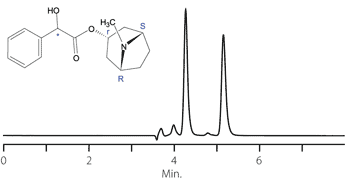
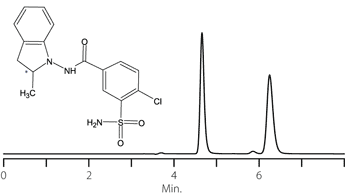
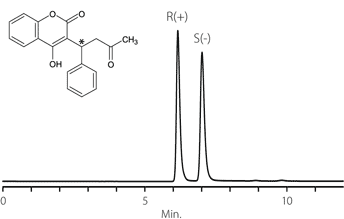
Figure 15. Ketoconazole
column: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 μm particles (51098AST)
mobile phase: acetonitrile
flow rate: 0.5 mL/min.
temp.: 25 °C
det.: UV, 230 nm
inj.: 2 μL
sample: ketoconazole (2 mg/mL in mobile phase)
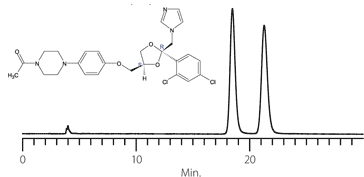
Figure 16. Stable Performance After Repeated NP-POM Cycles
columns: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 μm particles (51098AST)
mobile phase (normal phase): 10:90, IPA:heptane
mobile phase (POM): 0.1% w/v ammonium formate in methanol
flow rate: 0.5 mL/min.
temp.: 25 °C
sample: TSO (normal phase) or mianserin (POM)
det.: UV, 254 nm
inj.: 2 μL
SFC Chiral Separations
SFC (supercritical fluid chromatography) is gaining in popularity, especially for chiral separations, due to its speed advantages over HPLC. The CO2 is readily removed from the eluate, which makes it ideal for prep. DMPC-derivatized cellulose is widely used for chiral separations by SFC, both analytical and prep. The Astec Cellulose DMP works well in SFC mode, providing rapid separations with excellent selectivity. For example, Figure 17 shows the SFC separation of a mixture of six diastereomers of a single compound in less than four minutes on Astec Cellulose DMP. As a testament to its utility as a screening tool, the durability of Astec Cellulose DMP permits rapid (ballistic) gradients of methanol, ethanol or IPA in CO2 with long column lifetime and low backpressure, but without significant column bleed (Figure 18).
Figure 17. Rapid SFC Separations
column: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 μm particles (51098AST)
mobile phase: 10% ethanol in CO2
flow rate: 3 mL/min.
temp.: 35 °C
pressure: 100 bar
det.: UV, 220 nm
inj.: 5 μL
sample: six diastereomers of a single compound (proprietary drug substance)
Figure 18. Stability and Resolution under Ballistic SFC Gradients
column: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 μm particles (51098AST)
mobile phase: 10% ethanol in CO2
gradient: 5-65% methanol in CO2; hold 1 min.flow rate: 4 mL/min.
temp.: 35 °C
pressure: 100 bar
det.: UV, 220 nm
inj.: 5 μL
sample: warfarin, 2 mg/mL

SFC data for Figures 17 and 18 kindly provided by Dr. Christina Kraml, Lotus Separations, LLC, Princeton, NJ.
Ideal for Preparative Applications
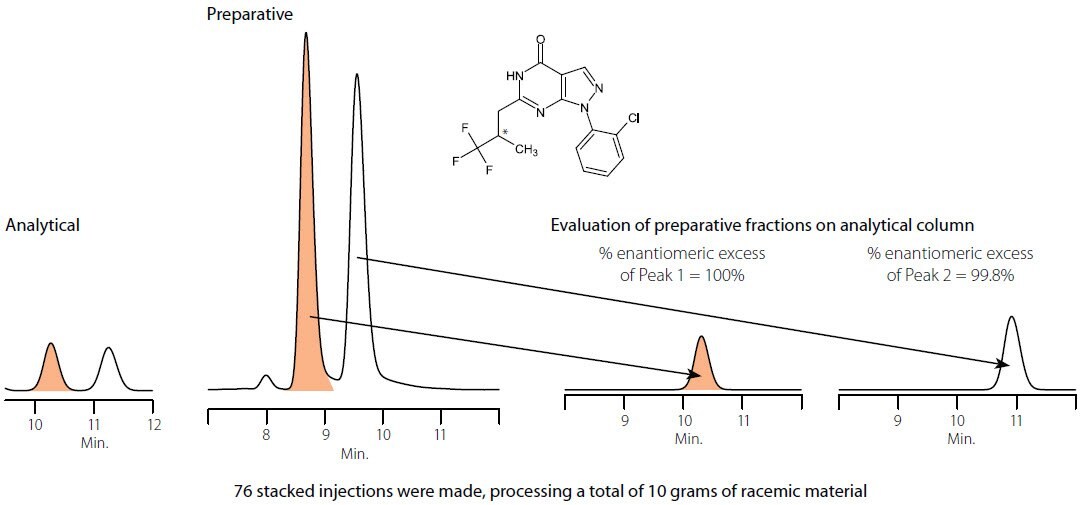
A characteristic of the polysaccharide-based CSPs that has contributed to their popularity is their utility for preparative applications. Although there are many factors to consider in a preparative scale up, the particle contribution comes primarily from the amount of available stationary phase and to what degree it resolves the enantiomers. When we designed the Astec Cellulose DMP, we sought to achieve the high sample loading and throughput that chiral chromatographers have come to expect. The sample loading (mg per injection) of Astec Cellulose DMP is comparable to Competitor D (Figure 19). An example of the scale-up of an analytical separation (4.6 mm I.D. column) to a preparative scale (21.2 mm I.D.) is shown in Figure 20 for the anti-Alzheimer's drug BAY 73-6691.
Figure 19. Loading Capacity
columns: 15 cm x 4.6 mm I.D., 5 μm particles
mobile phase: 10:90, IPA:hexane
flow rate: 1 mL/min.
temp.: 28° C
det.: UV, 210 nm
inj.: 100 μL
sample: trans-stilbene oxide (TSO), 0.05 – 50 mg/mL
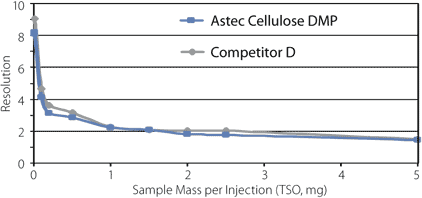
Figure 20. Scale-Up Example
columns: Astec Cellulose DMP, 5 μm particles
mobile phase: 80:10:10, heptane:methyl tert-butyl ether (MTBE):ethanol
temp.: 25 °C
det.: UV, 230 nm
sample: BAY 73-6691 in mobile phase
Analytical:
dimensions: 15 cm x 4.6 mm I.D. (51098AST)
flow rate: 0.5 mL/min.
inj.: 10 μL (2 mg/mL)
Preparative:
dimensions: 25 cm x 21.2 mm I.D. (51103AST)
flow rate: 13 mL/min.
inj.: 5000 μL (3.3 mg/mL)
Complementary to the Other Astec CSPs
Cellulose DMP is the latest addition to the Astec line of high-quality chiral stationary phases manufactured by Sigma-Aldrich. Other Astec HPLC CSPs include CHIROBIOTIC, CYCLOBOND, P-CAP, P-CAP-DP, CLC-L, and CLC-D. Astec CHIRALDEX and Supelco DEX are the market leaders for chiral GC separations. The HPLC CSPs are complementary to each other in terms of selectivity and mobile phase compatibility. This means:
- It is likely that at least one Astec CSP will give the necessary selectivity. Incorporating Astec Cellulose DMP, CHIROBIOTIC and CYCLOBOND in your HPLC or SFC screening protocol will give at least 90% success rate.
- Multiple Astec CSPs may provide the necessary enantioselectivity, but one may operate in a preferred mobile phase system, one that is more compatible with the detection mode, or provides better analyte solubility or shorter retention time, or many other considerations. For example, polar ionic CHIROBIOTIC mobile phases are ideal for LC/ESI-MS.
- Different CSPs may provide reversal of elution order, a useful attribute for prep and for low-level detection of the presence of an unwanted enantiomer in large excess of the opposite enantiomer (trace analysis).
The wide choice of CSPs in the Astec line means they cover many different areas of interest within chiral chromatography. Some of these areas are captured in Table 3. View the complete product listing of Astec Cellulose DMP columns.
References
To continue reading please sign in or create an account.
Don't Have An Account?