HybridSPE®-Phospholipid Technology
Effective Removal of Phospholipids and Proteins for Accurate and Reproducible LC-MS Analysis
Impact of Phospholipids on Accuracy and Reproducibility of LC-MS Data
Limitations of Traditional Sample Prep Methodologies for Biological Samples
The Unique Chemistry and Basic Methodology of HybridSPE®-phospholipids Technology
HybridSPE®-PLus Plates - the Next Generation in HybridSPE®-Phospholipid Technology
Our new HybridSPE®-PLus plates offer the same time-proven chemistry of our traditional HybridSPE®-Phospholipid plates coupled with plate manufacturing enhancements that stem from years of production experience. These manufacturing enhancements have allowed us to increase well-to-well flow consistency and reduce sample
hold-up volumes, further improving analyte recoveries and assay reproducibility. We will continue to offer and support our traditional square-well HybridSPE®-Phospholipid plates for those customers who have developed methods on them, but we invite those customers developing new methods to take advantage of the newest technology: HybridSPE®-PLus.
Impact of Phospholipids on Accuracy and Reproducibility of LC-MS Data
Phospholipids: A Concern for LC-MS Analysis of Small Molecules in Biological Matrices
Phospholipids are present as a major component of all cell membranes. They are therefore present in all biological sample matrices including serum, plasma and whole blood and can be a problem in LC-MS analysis of small molecules because they often co-elute and ionize along with the analytes of interest. This co-elution results in ion suppression (an erroneous decrease) of the mass spec signal that can cause variability and impact LC-MS result accuracy (Figure 1).
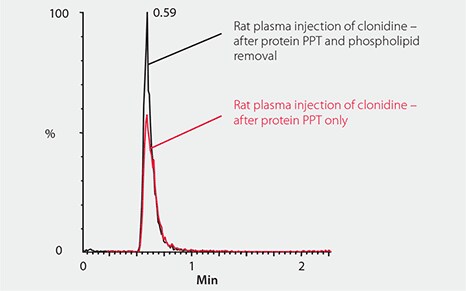
Figure 1. Phospholipid Effect on Ionization of Clonidine
Importance of Accurate Results and Fast Answers in Bioanalysis
We understand that the results of your analyses can have a significant impact on the lives of many others. This puts pressure on you to ensure the data you produce is as accurate as possible. Not only do you need quick answers, you need answers you can trust. The complexity of the types of samples with which you work does not make your job any easier. Proteins and phospholipids inherently present, to varying degrees in your samples, can add variability to your analytical results when using sensitive techniques such as LC-MS or LC-MS/MS. We have over four decades of chromatography expertise to help you navigate the complexity of your sample prep options.
Limitations of Traditional Sample Prep Methodologies for Biological Samples
Limitations of Traditional Biological Sample Cleanup Methods
Most clinical and biological researchers use traditional methods such as protein precipitation and liquid-liquid extraction to cleanup their samples prior to analysis. While these techniques allow for inexpensive and quick removal of proteins, they completely fail to address the problem of phospholipid-induced ion suppression.
Even if phospholipids do not co-elute with the analyte of interest, they can accumulate on your analytical column and elute from the column sporadically in downstream analyses. This can cause unpredictable ion suppression and poor reproducibility, thereby putting the accuracy of your results at risk (Figure 2).
Background Interference Observed after Multiple Injections Using Std. Protein PPT
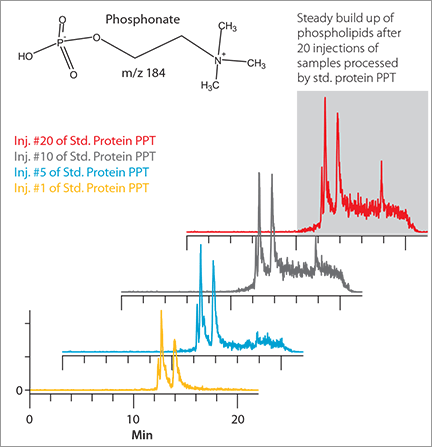
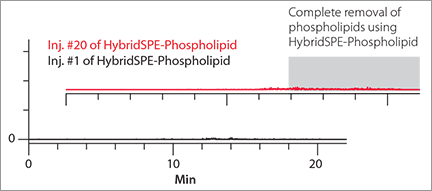
Figure 2. Gradient RP LC-MS of Blank Plasma Samples Prepared by Standard Protein PPT vs. HybridSPE®-Phospholipid
Background Interference Observed after Multiple Injections Using HybridSPE®-Phospolipid Technology
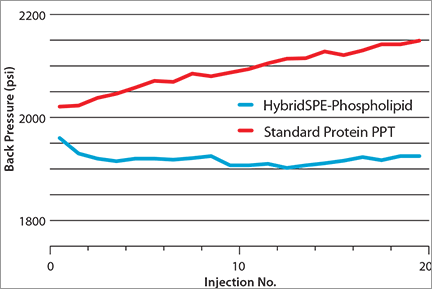
Back Pressure Generated Within C18 1.8 µm Column (5 cm x 2.1 mm) after Multiple Injections
Phospholipid Removal Techniques
To overcome the problem of phospholipid-induced ion suppression, some analysts try traditional SPE. Traditional SPE often requires time-consuming and complex method development, but still only removes nominal amounts of phospholipids. Remaining phospholipids can impact the accuracy of your results, accumulate on your analytical column to impact future analyses, add to column replacement costs and increase instrument downtime.
A variety of products designed specifically for the removal of both proteins and phospholipids are now commercially available, including HybridSPE® plates and cartridges. Most of these products are simple, fast and easy-to-use, offering fairly standardized methods with minimal method development. Most, however, use a hydrophobic retention mechanism to separate phospholipids from analytes of interest in the sample. This poses a problem if the analytes of interest are also hydrophobic because they will be retained and removed along with the hydrophobic phospholipids. This results in decreased analyte recovery and inaccurate results. HybridSPE®Phospholipid Technology is different in that it completely removes both proteins and phospholipids from the sample without retaining other hydrophobic compounds.
The Unique Chemistry and Basic Methodology of HybridSPE®-Phospolipid Technology
How Is HybridSPE®-Phospholipid Technology Different Than Other Phospholipid Removal Products?
The first of its kind, HybridSPE®-Phospholipid technology was introduced in 2008. It fuses the simple, standardized methodology of traditional protein precipitation with the specificity of solid phase extraction (SPE) for the simultaneous removal of proteins and phospholipids from biological samples prior to LC-MS analysis. Unlike other phospholipid removal products that use a hydrophobic retention mechanism to remove phospholipids from biological samples, HybridSPE®-Phospholipid technology uses a unique retention mechanism (Figure 3). This allows it to separate phospholipids from even very hydrophobic analytes, such as vitamins which are often retained along with phospholipids on competitive products.
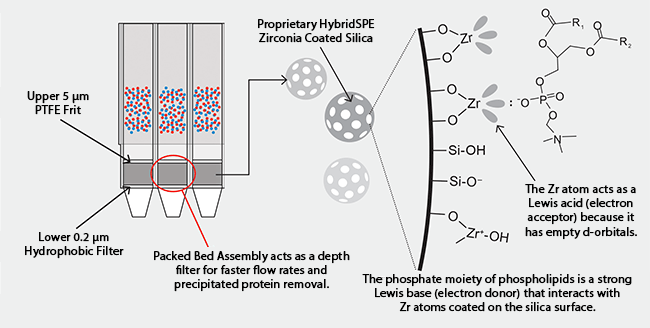
Figure 3. Unique Retention Mechanism of HybridSPE®-Phospholipid Technology Allows for Separation of Even Very Hydrophobic Analytes from Phospholipid Contaminants in Biological Sample Matrices
A Newer, Better “Go-to” Sample Prep Method for Phospholipid Removal
Labs working with biological samples often choose to perform protein precipitation prior to LC-MS analysis, using it as their “go-to” sample prep method. They view phospholipid removal as more costly, more time-consuming and unnecessary if the specific analytes of interest do not co-elute with the phospholipids in their sample. This is not the case.
Labs can actually reduce overall costs and increase overall throughput while generating more accurate data by using HybridSPE®-Phospholipid technology to remove both proteins and phospholipids from all biological samples prior to small molecule LC-MS analysis.
Simple and Fast Phospholipid Removal
The 96-well plate protocol involves just a few simple steps (Figures 4 and 5), and plates can be used right out of the package with no pre-conditioning step required. The sample (containing internal standards if desired) and a precipitation solvent are first added to the well plate, followed by a mixing step and vacuum application to collect the sample. Collected samples are then ready to be analyzed.
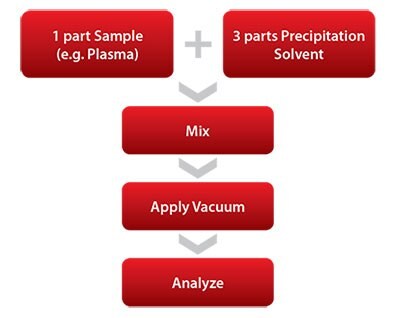
Figure 4. Depiction of Basic HybridSPE®-Phospholipid Sample Prep Workflow
1. Add Sample Pipette 100 µL plasma or serum to the HybridSPE® plate followed by 300 µL precipitation solvent. Add internal standards as necessary. |
2. Mix by vortexing/shaking HybridSPE® plate or by aspirating/dispensing with 0.5–1 mL pipette tip. |
3. Apply vacuum The packed-bed filter/frit assembly acts as adepth filter for the concurrent physical removal of precipitated proteins and chemical removal of phospholipids. Small molecules (e.g., pharma compounds and metabolites) pass through unretained. |
4. Collect Sample Resulting filtrate/eluate is free of proteins and phospholipids and ready for immediate LC-MS/MS analysis. |
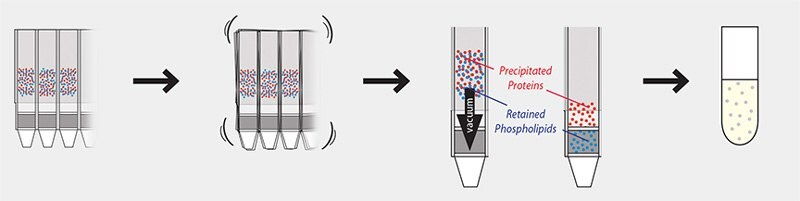
Figure 5. HybridSPE® 96-well Plate Protocol
Technical Resources |
|---|
To continue reading please sign in or create an account.
Don't Have An Account?