Extracellular Matrix Proteins and Tools for Cell Culture Optimization
Abstract
Animal cells and tissue culture techniques are constantly improved to optimize in vitro cell culture conditions. Extracellular Matrix (ECM) proteins coating, chemical or physical modification of the cell culture vessel, have proven to be efficient methods to better mimic in vivo cell behavior. We describe here the different coating available, with some new technologies highlights.
Background In 1900’s, animal tissues were cultured on glass surfaces, but as they require careful cleaning procedures, researchers started experimenting with disposable plastic culture vessels made up of polystyrene.1,2 However, plastic culture vessels have a certain number of limitations:3
- Difficulty in cell growth and cell attachment in serum-free media
- Change in cell shape, polarity and morphology
- Increase cell proliferation and decreased differentiation
- Less reactive to hormones and growth factors
Researchers then began coating vessel with both biological materials (biological coating) and synthetic polymers (chemical coating) that can enhance cell attachment, growth and differentiation. The growth of cells on coated surfaces is a more relevant representation of natural environment as contrary to the growing cells on flat, 2D plastic surfaces. This technique can be qualified as Physiological 2D environment, or 2.5D cell culture condition.
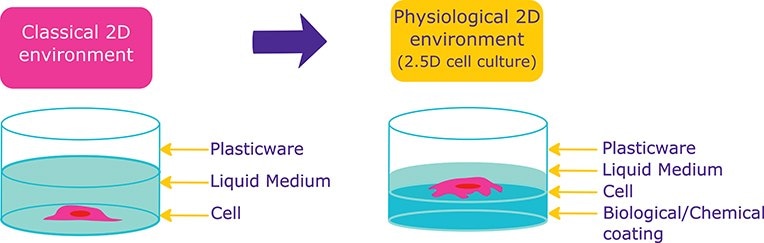
Figure 1.Cells in presence of Extracellular matrix proteins undergo physiologically relevant behaviours than cells in 2D conditions.
Cell cultured in presence of biological (extracellular matrix proteins) or chemical (Poly-Lysin…) coating are undergoing more physiologicaly relevant behaviors than cells cultured in classical, 2D cell culture conditions.
Components of the Extracellular Matrix
Tissues are not just tightly packed with cells; most of the volume contains extracellular space and is filled with complex meshwork of proteins called the extracellular matrix (ECM). The components of ECM in most tissues are secreted by fibroblasts and are categorized into proteoglycans and fibrous proteins (Collagen, elastin, fibronectin and laminin).4 These components give structural support and facilitate cellular communication. Integrins, the transmembrane proteins on the cell surface that link the cytoskeleton of cells to the ECM, activate signaling pathways that regulate cell proliferation, morphology, adhesion and cell death.
Collagen
Collagen is the most abundant protein in mammals, constituting 25% of total protein mass. It is composed of three polypeptide chains (designated alpha chains) arranged in a helical conformation, rich in glycine and proline residues. There are more than 20 different types of collagens, of them collagen I, II, III, V and XI are fibrillar collagen commonly found in connective tissue. Collagen type IX and XII are fibril-associated collagen, which link fibrils to one another and to the components of extracellular matrix. While, collagen type IV and VII are network-forming collagen that constitutes major part of basal lamina.5
In tissues, collagen provides structural support, strength and resilience, and in cell culture it is used to study growth, differentiation and migration of cells.6
Collagens available, with cell lines used and area of research
Elastin
Elastin is hydrophobic protein of 750 aminoacids, rich in proline and glycine. However, unlike collagen, these amino acid residues are not glycosylated. Tropoelastin, a soluble precursor secreted into the extracellular space assembles into insoluble elastic fibers and sheets. Elastic fibers provide required resilience so that tissues can recoil after transient stretch5.
Elastins available with cells lines used and and area of research
Fibronectin
Fibronectin is a large glycoprotein (220 kDa) composed of two polypeptide chains (dimer) joined by disulfide bonds at one end. Each polypeptide is further folded into functionally and structurally distinct domains which bind to various components of ECM (glycosaminoglycans, proteoglycans, and collagen) and cell surface proteins. Fibronectin is secreted by wide variety of connective tissue cells, including: fibroblasts, chondrocytes, schwann cells, macrophages, intestinal epithelial cells and hepatocytes7.
Fibronectin is a multifunctional protein involved in cell adhesion and spreading. It also regulates cellular morphology, cell migration, cytoskeletal organization, hemostasis and wound repair.
Fibronectins available, with cell lines used and area of research
Searching for a recombinant, animal component free fibronectin? Try human recombinant fibronectin.
Laminin
Laminin is major component of basal lamina. It is composed of three long polypeptide chains (designated α, β, and γ) held together by disulfide bonds and arranged in asymmetric cross shape. Laminin acts as glue, which holds cells and ECM together. It has active domains for collagen binding, cell adhesion, heparin binding and neurite outgrowth fragment. Laminin modulate cell growth, motility and signaling pathways3,8.
Laminins available, with cells lines used and area of research.
Vitronectin
Vitronectin is glycoprotein of 459 aminoacids, found in ECM and blood. It circulates in blood either in the form of single chain moiety of 75kDa or as two chain moiety of 65kDa and 10kDa. Vitronectin interacts with polysaccharides (Glycosaminoglycans) and proteoglycans, acting as cell adhesion molecule. Although, vitronectin and fibronection have similar functions and have an Arg-Gly-Asp cell recognition sequence, they are structurally and immunologically distinct.9
Vitronectin acts as inhibitor of cytolytic complement pathway and have physiological role in coagulation pathway. In addition, it promotes cell migration, proliferation, differentiation and spreading of endothelial and neoplastic cells.
Vitronectins available, withcells lines used and area of research
Not sure what is the best suited ECM protein for your cell or application?
Our ECM cell culture optimization array enables to identify the best ECM protein and also pinpoint the concentration needed to achieve optimal cell growth conditions.
Need to optimize a specific ECM protein concentration for a new cell/application?
Try the Millicoat™ cell adhesion strips, provided as 12 removable 8-well strips in a plate frame. The wells in rows A-G are precoated with an ECM protein and row H is coated with BSA to serve as a negative control.
ECM peptides
Extracellular matrix components provide both biochemical and physical cues for cellular functions. The current technology merely offers adequate environment for simple cellular process like cell attachment. But a recent study showed that combination of these extracellular matrix derived peptides on surface improve cell proliferation rate, cell adhesion strength and focal adhesion assembly.10 Kollodis BioSciences’ MAPTrix™ Technology provides a true ECM microenvironment by incorporating combinatorial peptide motifs to induce and/or regulate combinatorial integrin mediated signaling processes. The Kollodis ECM Library provides a means to regulate a variety of cell surface receptors, this technology replaces traditional ECM peptides with genetically incorporated peptides into mussel adhesive protein that maintain cells under serum and feeder free conditions.
Chemical/synthetic coatings
Poly-Lysine and poly-ornithine
Coating of synthetic polymers (poly amino acids) facilitates the attachment of both cells and proteins. Poly-amino acids like poly-lysine and poly-ornithine create a positive charge on polystyrene and increase the positively-charged sites available for cell binding.11 They are also used in combination with attachment factors which can promote electrostatic interaction between negatively-charged ions on the cell membrane and positively-charged ions of attachment factors on the culture surface.
Products used forchemical coating, with cells line used and area of research
Cytosoft coating
The rigidity of the substrate influences the cellular functions. CytoSoft® plates are coated with thin layer of biocompatible silicone, with various rigidities covering a broad physiological range. The surface of the gels forms stable covalent bonds with proteins, facilitating the coating of the gel with attachment factors (ECM components) and plating cells. Following are the advantages of CytoSoft® plates:
- Optically clear and have low auto-florescence
- Silicone gels are not susceptible to hydrolysis
- Silicone gels are stable, they do not dry nor swell
- Resistant to tearing or cracking
- Rigidities nearly unchanged during extending storage periods
- Trypsin and collagenase can be used to harvest cells
- Resistant to biochemical breakdown after enzyme treatment
Conclusion
Coating of culture surface with ECM proteins and synthetic polymers greatly influences cell behavior. The response observed is dependent on both cell type and coating used as substrate. Cells in contact with the attachment factors survive longer and can also be grown in absence of serum factors.3 The attachment factors can sequester and store growth factors, controlling spatio-temporal regulation of factors and facilitates cross talk between growth factor receptors and ECM receptors. It also defines mechanical properties and instructs cells to differentiate under permissive conditions. ECM proteins also induce intracellular signaling through cell-surface receptor in synergy with growth factor signaling.12 As cell culture is evolving, more components and combinations are needed to better mimic the in-vivo conditions of tissues and decipher the language of extracellular matrix between cells.
To continue reading please sign in or create an account.
Don't Have An Account?