Nicewicz Photoredox Catalysts for Anti-Markovnikov Alkene Hydrofunctionalization
Introduction
While Markovnikov alkene reactivity is very well developed and utilized commonly in the synthesis of commodity and research chemicals, catalytic access to the anti-Markovnikov-selective adducts is a much less-developed endeavor. In collaboration with Professor David Nicewicz, Sigma-Aldrich is proud to offer a selection of photoexcitable acridinium salts that can facilitate various olefin hydrofunctionalizations with complete anti-Markovnikov selectivity.
The cationic organic oxidant 9-mesityl-10-methylacridinium perchlorate was originally designed by Shunichi Fukuzimi and co-workers.1 Since its introduction, the Nicewicz group has demonstrated the wide utility of this material and related photoredox catalysts (794171, 793876, 793221) when used in conjunction with primarily thiol-containing hydrogen atom transfer co-catalysts.2 Through the generation of alkene cation radical intermediates,3 this unique photoredox catalyst system affords hydrofunctionalization of an expansive range of activated and unactivated alkenes with diverse nucleophiles, such as carboxylic acids, amines, mineral acids, and propargylic and allylic alcohols.2–3
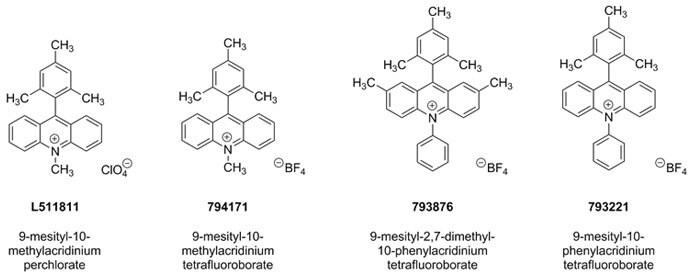
Figure 1.Propargylic-and-allylic-alcohols
Advantages
- Metal-free, visible light-mediated catalyst
- Stable to air and moisture
- Mild reaction conditions
- Anti-Markovnikov selectivity
- Superior oxidizing capabilities compared to Ru- and Ir-polypyridyl photoxidants
- Straightforward excitation of acridinium salts with LED flood lights
Representative Applications
Hydroetherification
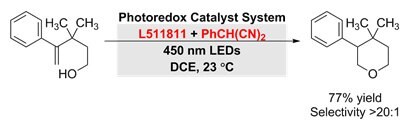
Complete regioselectivity was observed in over 10 examples of direct intramolecular anti-Markovnikov hydroetherification of alkenols when 9-mesityl-10-methylacridinium perchlorate was used with 2-phenylmalononitrile as a hydrogen atom donor.4
Hydroamination
Hydroamination of alkenes can be accessed using 9-mesityl-10-methylacridinium tetrafluoroborate (794171), with complete anti-Markovnikov selectivity in the presence of a thiol-containing co-catalyst. With thiophenol, intramolecular hydroamination of unsaturated amines provided access to several nitrogen-containing heterocycles.5 Furthermore, intermolecular hydroamination of aliphatic alkenes, α- and β-substituted styrenes, and heterocyclic amines were achieved with diphenyl disulfide.6
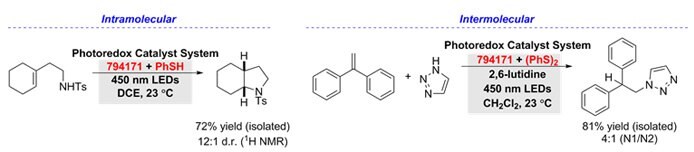
Figure 2.Diphenyl-disulfide
Hydrotrifluoromethylation
Diverse trifluoromethylated products have been obtained through this photoredox catalyst system. Single electron oxidation of the Langlois reagent (743232) by 9-mesityl-10-methylacridinium tetrafluoroborate (794171) and a thiol-containing co-catalyst (methyl thiosalicylate or thiophenol) results in the hydrotrifluoromethylation of styrenes and unactivated aliphatic alkenes with anti-Markovnikov selectivity. Nicewicz and co-workers provided 20 examples with 25–74% yields.7

Figure 3.Hydrotrifluoromethylation-of-styrenes
Hydroacetoxylation
Anti-Markovnikov-selective hydroacetoxylation of styrenes, enamides, and trisubstituted aliphatic amines is possible with a range of carboxylic acids when using 9-mesityl-10-methylacridinium tetrafluoroborate (794171) in combination with sodium benzene sulfinate or thiophenol. Seventeen reactions exhibited yields ranging from 29–99%.8

Figure 4.Sodium-benzene-sulfinate
Addition of Mineral Acids
The anti-Markovnikov addition of strong Brønsted acids to alkenes is achieved with complete regioselectivity using these photocatalysts. For hydrochlorination, 9-mesityl-10-methylacridinium tetrafluoroborate (794171) is used with thiophenol to generate the adduct. Hydrofluorination, hydrooxyphosphorylation and hydrooxysulfonylation are each effected by employing 9-mesityl-2,7-dimethyl-10-phenylacridinium tetrafluoroborate (793876) with the hydrogen-atom donors 4-nitrophenyl disulfide or 4-methoxythiophenol. Here, the inclusion of 2,6-lutidinium salts were found to provide the best reactivity through a nucleophilic couterion.9
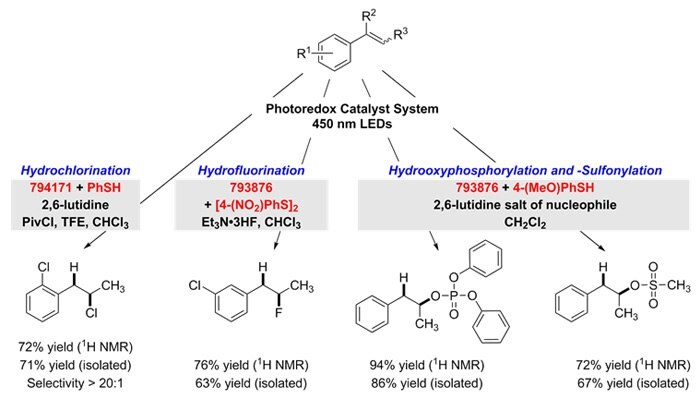
Figure 5.Nucleophilic-couterion
Intramolecular Hydrofunctionalization of Amides
The anti-Markovnikov intramolecular cyclization of unsaturated allylic amides and thioamides furnishes the resultant 2-oxazolines and 2-thiazolines, respectively. These transformations utilize a photoredox catalyst system comprising 9-mesityl-10-methylacridinium tetrafluoroborate (794171) in combination with diphenyl disulfide. Through 17 examples, a range of 59–82% yields were observed.10

Figure 6.Combination-with-diphenyl-disulfide
Hydrodecarboxylation
The anti-Markovnikov hydrodecarboxylation of carboxylic and malonic acids is accomplished with 9-mesityl-10-phenylacridinium tetrafluoroborate (793221) and diphenyl disulfide. The decarboxylation reaction requires the inclusion of a base and a polar alcohol solvent, trifluoroethanol in order to afford good yields and scope. Eighteen examples were included to demonstrate the hydrodecarboxylation of primary, secondary, and tertiary carboxylic acids.11
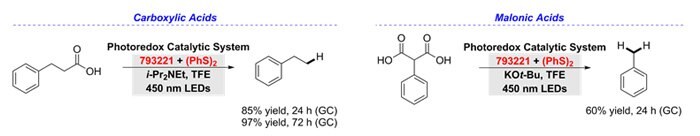
Figure 7.Tertiary-carboxylic-acids
Polar Radical Crossover Cycloadditions
In a tandem addition-cyclization sequence, polar radical crossover cycloadditions use variations of the photoredox catalyst system to generate the following products from alkenes: g-butyrolactones (with α,β-unsaturated carboxylic acids),12 tetrahydrofurans (with allylic alcohols),13 g-lactams (with unsaturated amides), and pyrrolidines (with unsaturated amines).14
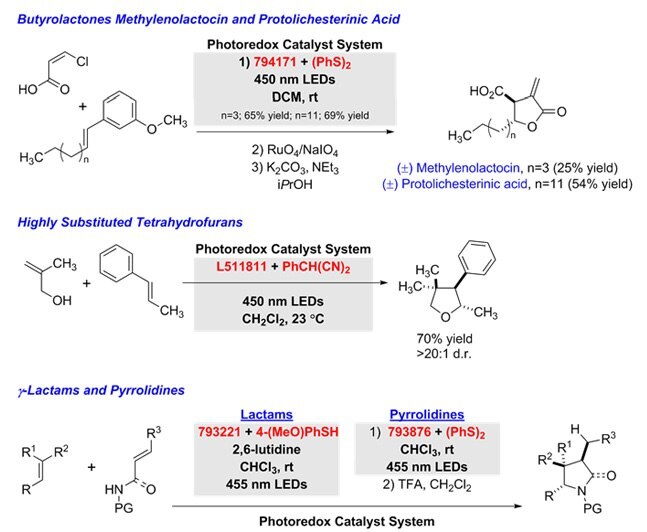
Figure 8.Unsaturated-amines
Photooxygenation
Prior to Nicewicz’s reports, Griesbeck and Cho used 9-mesityl-10-methylacridinium perchlorate in the presence of O2 as a visible-light mediated catalyst for Type II and electron-transfer photooxygenation reactions.15
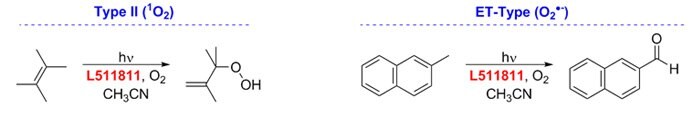
Figure 9.Photooxygenation-reactions
References
To continue reading please sign in or create an account.
Don't Have An Account?