Asymmetric Epoxidation Using Shi Catalyst
Daniel Weibel
Catalytic asymmetric epoxidation of alkenes has been the focus of many research efforts over the past two decades, the best known methods probably being those developed by Sharpless1 and Jacobsen-Katsuki.2 Shi has also developed a very efficient method for asymmetric epoxidation, using a ketone-derived organocatalyst based on d-fructose (F0127).3 This organocatalyst is able to epoxidize trans alkenes and certain cis alkenes with good to excellent yields and selectivities. More recently, Shi has achieved excellent results using hydrogen peroxide as an oxidant instead of OXONE® (228036), which allows a significant reduction in the amount of additional salts introduced and solvent used in the reaction (Scheme 1).4
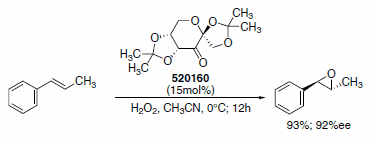
Scheme 1.Enantioselective epoxidation of trans alkenes using Shi organocatalyst (520160).
Other groups have used Shi’s methodology in pursuit of a number of unique structural moieties. For example, McDonald and co-workers recently reported a robust and selective synthesis of 2-amino-3,5-diols that employs the Shi epoxidation in a key step (Scheme 2).5 These 2-amino-3,5-diols are 1-deoxy- 5-hydroxysphingosine analogues, which show promise as anticancer agents.
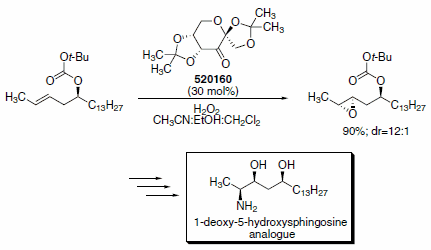
Scheme 2.Selective route to sphingosine derivatives using Shi’s epoxidation organocatalyst (520160)
A recent example of the use of the Shi catalyst is the synthesis of chiral thiosulfonate derivatives. Chiral sulfinyl derivatives have been of interest for the past three decades.6 This interest stemmed from the use of these molecules as chiral controllers in asymmetric synthesis, as chiral ligands and as Lewis bases. Khiar et. al. reported a new method to synthesize enantiomerically pure, functionalized, sterically demanding thiosulfonates.7 Investigating several catalysts for the oxidation of disulfides, diesters and diethers, Khiar et al. determined that a particular chiral pyranone derived from d-glucose, the Shi catalyst, yielded the best yields and stereoselectivities. Using the Shi catalyst in combination with oxone in acetonitrile and dimethoxymethane, the authors reported up to 89% yield and up to 96% ee for the oxidation of disulfides (Scheme 3).
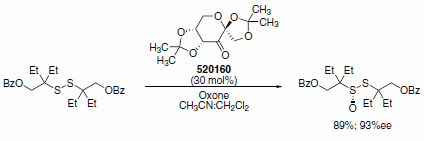
Scheme 3. Enantioselective disulfide oxidation using Shi catalyst (520160).
References
To continue reading please sign in or create an account.
Don't Have An Account?