- Products
- Physical Properties
- Specificity and Kinetics
- Inhibitors
- Substrates
- Solubility and Solution Stability
Physical Properties
Molecular Weight: 25 kDa1 (Bovine)
pI: 8.752 (Bovine)
Extinction coefficient: E1%= 20.4 (280nm)
Chymotrypsin is produced in the acinar cells of the pancreas as the inactive precursor, chymotrypsinogen. α-Chymotrypsin is the predominant form of active enzyme produced from it's zymogen, Chymotrypsinogen A.
In vivo, the rate of hydrolysis of the zymogen by trypsin and by autolysis produces varying amounts of α, π, δ and γ variants.3 α-Chymotrypsin is a serine protease of the peptidase S1 family consisting of 241 amino acid residues. The molecule has three peptide chains: an A chain of 13 residues, a B chain of 131 residues, and a C chain of 97 residues.4
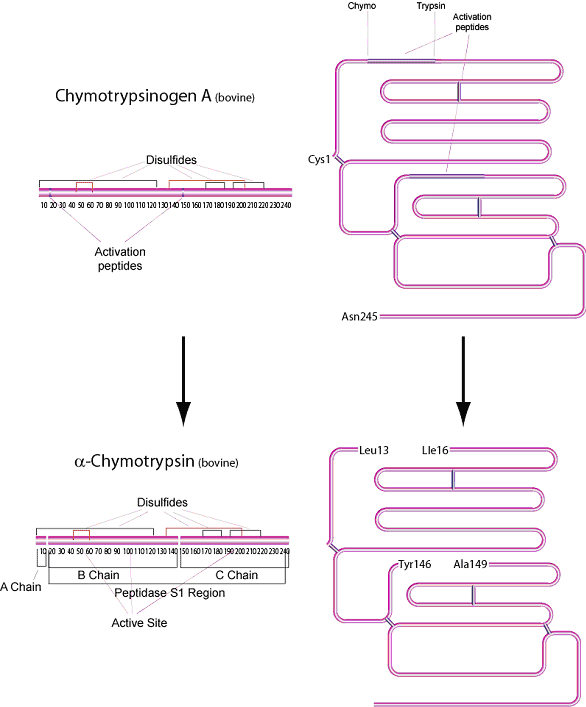
Figure 1.Physical Properties
Specificity and Kinetics
α-Chymotrypsin from bovine pancreas selectively catalyzes the hydrolysis of peptide bonds on the C-terminal side of tyrosine, phenylalanine, tryptophan, and leucine. A secondary hydrolysis will also occur on the C-terminal side of methionine, isoleucine, serine, threonine, valine, histidine, glycine, and alanine.1
pH optimum: 7.88
(pH 6.0: about 35% of maximal activity, pH 9.3: 40% of maximal activity)8
Temperature Optimum: 50 °C9
Solubility and Solution Stability
Chymotrypsin is typically soluble in 1 mM HCl (2 mg/mL), yielding a clear solution.
Reconstitute in 1 mM HCl containing 2 mM CaCl2, aliquot, and store at -20 °C. Autolysis will occur when stored at a higher pH. The presence of calcium is also a stabilizer.5 Frozen aliquots are stable for approximately 1 week. Chymotrypsin is both activated and stabilized by Ca2+. The enzyme is active in the presence of 0.1% SDS and 2 M guanidine hydrochloride.
Applications
For peptide digestion, use a ratio (w/w) of approximately 1:60 for chymotrypsin:peptide. Perform peptide digests in 100 mM Tris HCl containing 10 mM CaCl2, pH 7.8, at 30 °C. Self digestion may occur if temperatures above 37 °C are used. A known peptide such as melittin should be used as a control for all experiments. Incubate up to 24 hours at 37-40 °C. Digestion can be terminated by adjusting the pH to 2.0.6,7
References
To continue reading please sign in or create an account.
Don't Have An Account?