推荐产品
蒸汽密度
3.1 (vs air)
质量水平
蒸汽压
18 mmHg ( 21.1 °C)
产品线
ReagentPlus®
检测方案
99%
形式
liquid
环保替代产品特性
Less Hazardous Chemical Syntheses
Safer Solvents and Auxiliaries
Design for Degradation
Learn more about the Principles of Green Chemistry.
折射率
n20/D 1.368 (lit.)
bp
90 °C (lit.)
mp
2-4 °C (lit.)
密度
1.069 g/mL at 25 °C (lit.)
环保替代产品分类
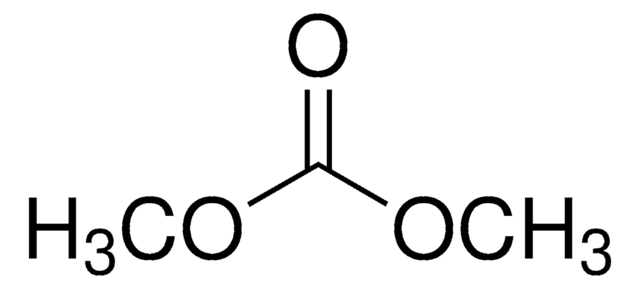
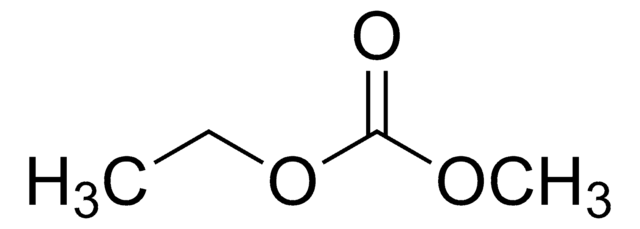
SMILES字符串
O=C(OC)OC
InChI
1S/C3H6O3/c1-5-3(4)6-2/h1-2H3
InChI key
IEJIGPNLZYLLBP-UHFFFAOYSA-N
正在寻找类似产品? Visit 产品对比指南
一般描述
我们致力于为您带来更环保的替代产品,以符合一项或多项绿色化学12项原则。此产品更加绿色环保,是传统溶剂和化学制剂的替代品。点击此处以获取更多信息。
应用
- 苯酚与碳酸苯酯的酯交换反应。
- 碳酸二苯酯与碳酸甲基苯酯通过酯交换反应生成碳酸二苯酯。
- 氨基甲酸甲酯,异氰酸酯合成的原料。
- 在 550-600K 下与二氧化硅反应生成四甲氧基硅烷。
特点和优势
法律信息
警示用语:
Danger
危险声明
危险分类
Flam. Liq. 2
储存分类代码
3 - Flammable liquids
WGK
WGK 1
闪点(°F)
60.8 °F - closed cup
闪点(°C)
16 °C - closed cup
个人防护装备
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
法规信息
分析证书(COA)
输入产品批号来搜索 分析证书(COA) 。批号可以在产品标签上"批“ (Lot或Batch)字后找到。
已有该产品?
为方便起见,与您过往购买产品相关的文件已保存在文档库中。
难以找到您所需的产品或批次号码?
如何查找产品货号
在网站页面上,产品编号会附带包装尺寸/数量一起显示(例如:T1503-25G)。请确保 在“产品编号”字段中仅输入产品编号 (示例: T1503).
示例
其它示例:
705578-5MG-PW
PL860-CGA/SHF-1EA
MMYOMAG-74K-13
1000309185
输入内容 1.000309185)
遇到问题?欢迎随时联系我们技术服务 寻求帮助
如何查找COA批号
批号可以在产品标签上"批“ (Lot或Batch)字后面找到。
Aldrich 产品
如果您查询到的批号为 TO09019TO 等,请输入去除前两位字母的批号:09019TO。
如果您查询到的批号含有填充代码(例如05427ES-021),请输入去除填充代码-021的批号:05427ES。
如果您查询到的批号含有填充代码(例如 STBB0728K9),请输入去除填充代码K9的批号:STBB0728。
未找到您寻找的产品?
部分情况下,可能未在线提供COA。如果搜索不到COA,可在线索取。
商品
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门