Lopinavir and Ritonavir Tablets Assay Method Following United States Pharmacopeia Monograph Guidelines Using an Ascentis C8 Column and UV Detection
Introduction
This application note illustrates how it is possible to set-up an assay method for Lopinavir and Ritonavir tablets. The given experimental conditions follow the current USP43-NF38 monograph; the chromatographic assay method (HPLC-UV) is isocratic in nature and calls for an L7 column (octyl silane; C8 modification). The method has been validated following the guidelines in USP General Chapters <621>, <1225>, and <1226>.
Lopinavir and Ritonavir were baseline resolved (Rs > 5) within 40 minutes using an Ascentis C8 HPLC column (150 x 4.6 mm, 5 µm) with a mixture of 4.1 g/L monobasic potassium phosphate solution, acetonitrile, methanol, and tetrahydrofuran as the mobile phase. Under the applied conditions ritonavir elute prior to lopinavir, and all system suitability requirements are met. The method demonstrates good selectivity, reproducibility, sensitivity and accuracy.
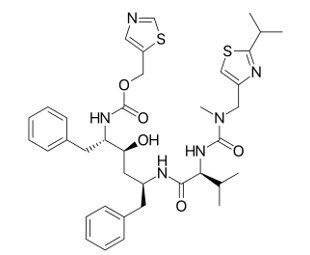
Figure 1.Ritonavir
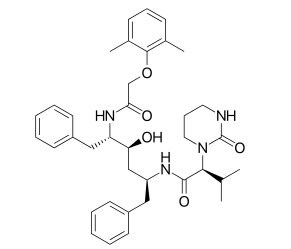
Figure 2.Lopinavir
Experimental Conditions |
|---|
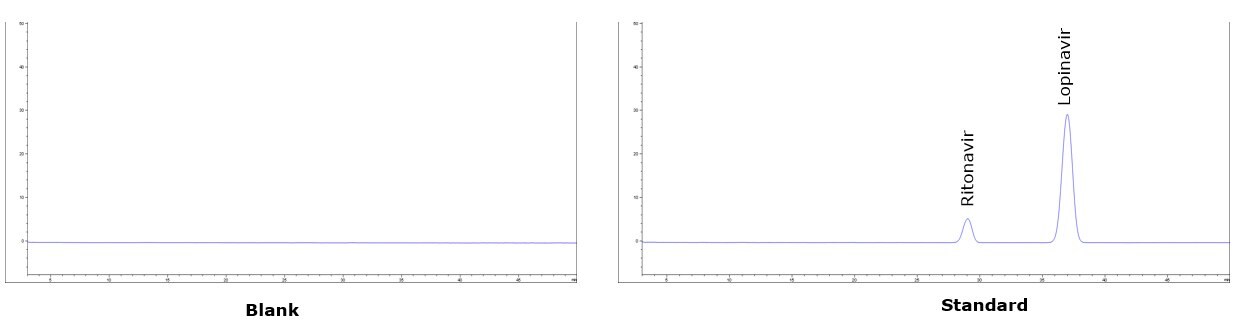
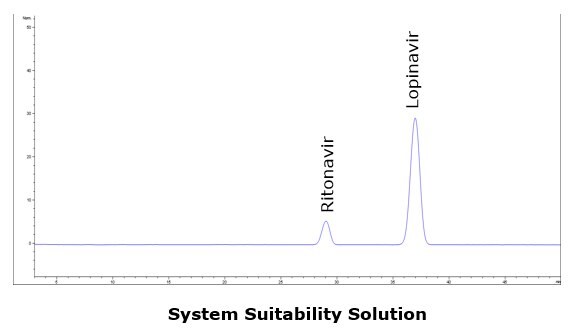
Chromatographic Data (System Suitability Solution)
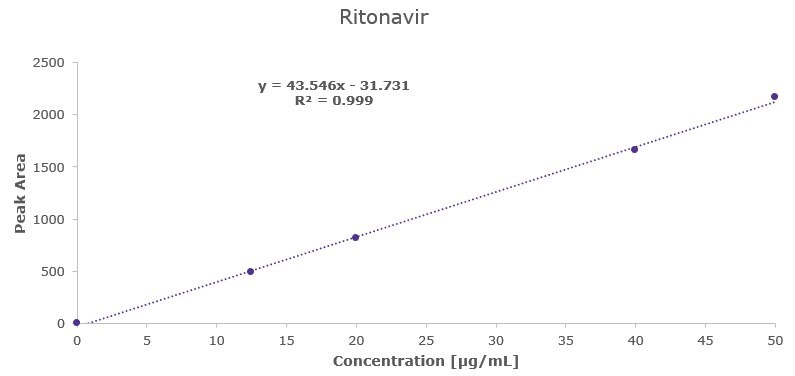
Ritonavir Linearity
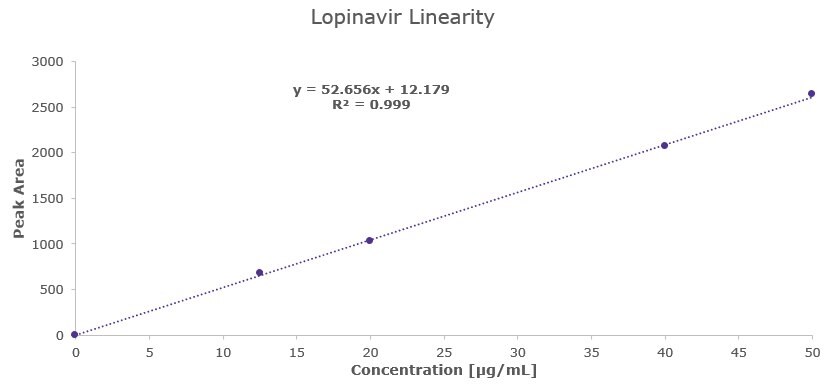
Lopinavir Linearity
如要继续阅读,请登录或创建帐户。
暂无帐户?