Fluorinating Reagents
The importance of selectively fluorinating compounds in medicinal chemistry, biology, and organic synthesis is well appreciated and provides a major impetus to the discovery of new and mild fluorinating agents that can operate safely and efficiently. Elemental fluorine and many electrophilic fluorinating agents have been used in synthesis; however, most of these fluorinating agents are highly aggressive, unstable, and require special equipment and care for safe handling. Sigma-Aldrich is pleased to offer the following alternatives, which lack these drawbacks.
4-Iodotoluene Difluoride (Tol-IF2)
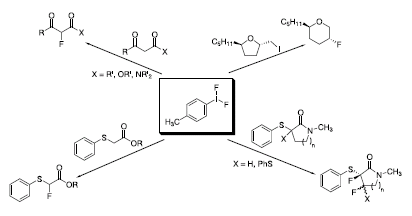
4-Iodotoluene difluoride (Tol-IF2) is easy to handle and less toxic than many fluorinating agents. Selective monofluorination of b-keto esters, b-keto amides, and b-diketones takes place under mild conditions without the use of HF–amine complexes.1 A new methodology for the synthesis of fluorinated cyclic ethers was recently reported, which utilized Tol-IF2 to achieve a fluorinative ring-expansion of four-, five-, and six-membered rings.2 When one equivalent of Tol-IF2 is reacted with phenylsulfanylated esters, the afluoro sulfide results through a fluoro-Pummerer reaction.3 When phenylsulfanylated lactams were treated with two equivalents of Tol-IF2, the lactams were fluorinated in the a and b positions, resulting in diastereomeric difluorides.4
Selectfluor™ Fluorinating Reagent (F-TEDA)
Selectfluor™ fluorinating reagent [(1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2] octane bis(tetrafluoroborate), or F-TEDA)] is a user friendly, mild, air- and moisturestable, non-volatile reagent for electrophilic fluorination. Selectfluor™ is capable of introducing fluorine into organic substrates in one step, with a remarkably broad scope of reactivity, often with excellent regioselectivity.5 For example, allylic fluorides can be prepared using Selectfluor™ via a sequential cross-metathesis–electrophilic fluorodesilylation route. This route avoids the formation of byproducts that result from allylic transposition, which is observed when nucleophilic displacement or ring-opening reaction with DAST is attempted.6
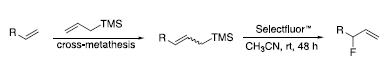
References
如要继续阅读,请登录或创建帐户。
暂无帐户?