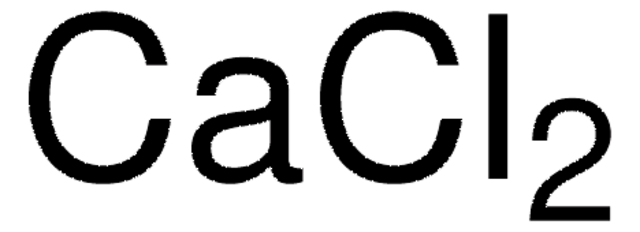CAPHOS
Calcium Phosphate Transfection Kit
Most cost effective transfection reagent kit for transient and stable transfection of DNA into mammalian cells
Recommended Products
grade
for molecular biology
Quality Level
form
solution
usage
kit sufficient for 160 transfections (6 cm dishes)
kit sufficient for 400 transfections (3.5 cm dishes)
kit sufficient for 80 transfections (10 cm dishes)
technique(s)
transfection: suitable
shipped in
dry ice
storage temp.
−20°C
Related Categories
General description
Application
- to enable transfection
- to transfect Hek293T cells
- to transfect H29D cells
BAEC
Bowels melanoma cells
CHO K1
COS-7
Fibroblasts (human embryonic, neo derm)
HEK293
Huh 7
IMR-90
LLC (Lewis Lung Carcinoma)
NIH3T3
PC-12
PCI-13
SH-Sy5Y
SK-Hep-1
T47D
Features and Benefits
- Suitable for transient and stable transfection
- Reproducible for a wide range of cell types
- Widely referenced
- Inexpensive
Components
5 ml 2.5M CaCl2 (C2052)
25 ml 2x HEPES Buffered Saline (H1012)
25 ml molecular biology grade water (W4502)
Principle
Other Notes
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Documents related to the products that you have purchased in the past have been gathered in the Document Library for your convenience.
Difficulty Finding Your Product Or Lot/Batch Number?
How to Find the Product Number
Product numbers are combined with Pack Sizes/Quantity when displayed on the website (example: T1503-25G). Please make sure you enter ONLY the product number in the Product Number field (example: T1503).
Example:
Additional examples:
705578-5MG-PW
PL860-CGA/SHF-1EA
MMYOMAG-74K-13
1000309185
enter as 1.000309185)
Having trouble? Feel free to contact Technical Service for assistance.
How to Find a Lot/Batch Number for COA
Lot and Batch Numbers can be found on a product's label following the words 'Lot' or 'Batch'.
Aldrich Products
For a lot number such as TO09019TO, enter it as 09019TO (without the first two letters 'TO').
For a lot number with a filling-code such as 05427ES-021, enter it as 05427ES (without the filling-code '-021').
For a lot number with a filling-code such as STBB0728K9, enter it as STBB0728 without the filling-code 'K9'.
Not Finding What You Are Looking For?
In some cases, a COA may not be available online. If your search was unable to find the COA you can request one.
Which document(s) contains shelf-life or expiration date information for a given product?
If available for a given product, the recommended re-test date or the expiration date can be found on the Certificate of Analysis.
How do I get lot-specific information or a Certificate of Analysis?
The lot specific COA document can be found by entering the lot number above under the "Documents" section.
How do I find price and availability?
There are several ways to find pricing and availability for our products. Once you log onto our website, you will find the price and availability displayed on the product detail page. You can contact any of our Customer Sales and Service offices to receive a quote. USA customers: 1-800-325-3010 or view local office numbers.
What is the Department of Transportation shipping information for this product?
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Why do I see a precipitate in my cell culture after calcium-phosphate transfection?
The precipitate is normal to see - this is the calcium/DNA precipitate the cell will internalize during the transfection process. If bubbled correctly, the precipitates will be very fine and regular sized, then evenly distributed over the cells.
Is low cell passage number an important consideration for transfection?
Yes, we recommend cells are at a low passage when being used for any application, including transfection. The reason why depends on what type of cells they are. Primary cells will undergo a finite number of divisions, and as they get closer to senesence they divide more slowly - both affecting their ability to take up DNA (transient transfection), and minimizing their abillity to incorporate the DNA into the genome (stable selection).Cultured common cell lines are often immortalized, and generally continue to aquire mutations, leading to a heterogenous population that may perform differently from cells of lower passage number - leading to results that are not reproducible.
Is the size of the plasmid an important consideration for transfection?
The size of the plasmid should be considered when selecting a transfection reagent with the best efficiency. In general, larger sized plasmids should easily transfect with readily available transfection reagents, as along as the plasmid DNA is of high purity.
Is optimizing the transfection protocol important?
For many common cell lines, transfection reagent efficiency is very high and the protocols will not require any optimization. For hard-to-transfect cells or those ultimately expressing a toxic protein, the protocol should be optimized for best transfection efficiency. Taking time to optimize will give you more transfected cells with each procedure, which can mean more protein expressed and results that are reproducible.
How do I choose a transfection reagent?
There are many guides that help you select a transfection reagent. In general, consider:The type of cell(s) you will transfectThe type of nucleic acid or protein you will introduce to the cellThe composition of your cell culture mediumThe need for stable or transient transfectionThe equipment you have availableThe other factors important to you - cost, protocol flexibility, ease of use, etc.
What quality does the DNA need to be in order to use it for transfection?
The DNA needs to be good quality or it may cause the cells to lyse and/or they won't transfect efficiently. Plasmid DNA prepared with a column-based DNA purification kit is suitable for transfections. Sigma's GenElute™ Minprep, Midiprep and Maxiprep kits work well for DNA plasmid purification. After preparing the DNA, confirm the OD A260:A280 ratio is greater than 1.6 for use in plasmid transfections.
What is transfection efficiency?
Transfection efficiency is a measure of how many cells take up the DNA during the transfection process. Many transfection reagents can achieve a transfection efficiency of >90% in common cell lines. Other cell lines are hard to transfect, and require special reagents and/or techniques to achieve even a small population of transfected cells.
How can I determine the efficiency of my transfection?
Calculating transfection efficiency is very useful when optimizing transfection protocols. Transfection efficiency can be performed using a GFP-expressing plasmid. After transfection, cells are stained with propidium iodide and counted. The propidium iodide provides a count of the total cells in the population, and the GFP-expressing cells provide a count of the number of cells transfected. The transfection efficiency (%) can then be calculated by:(# GFP-expressing cells / total cell #) * 100
How can I increase the efficiency of my transfection?
Transfection efficiency is affected by many different things, including plasmid size and purity, media components present, transfection reagent selected, amount of DNA and transfection reagent used, cell density, etc. Optimizing the protocol with respect to these concerns will allow you to achieve a higher transfection efficiency. For many cell lines and transfection reagents, optimized protocols are already available.
Can I transfect cells plated at low density?
For most transfections, cells should be >70% confluency the day of transfection, and growing in mid-log phase. Some transfection reagents are now designed to work with cells at low density, when required.
Can antibiotics be present in the medium during transfection?
We recommend that no antibiotics are present during transfection. The process of transfection can make the cells somewhat more porous to allow for efficient DNA entry. During this time, antibiotics will also enter the cells more easily and the cells may show increased cell death. Wait until about 24 hours after transfection to resume the use of preventative antibiotics and/or start the use of selective antibiotics.
How can I increase the efficiency of the calcium phosphate transfection method?
The calcium phosphate transfection method is performed differently than other methods, and so it can be optimized beyond the general recommendations. An important part of the protocol is generation of a fine precipitate. Be sure that the bubbling in tube B is consistant and with good force, while the solution from tube A is added very slowly, dropwise. Once the precipitates are added to the cell culture, be sure they are evenly distributed on the plate so all cells may be transfected.The pH of the HeBS is critical to precipitate formation. Prolonged storage of the solution may cause the pH to shift away from the optimal 7.05 - 7.12.
What is the difference between stable and transient transfection?
When the DNA enters the nucleus of the cell, the plasmid is replicated by the cell machinery (transient transfection). During this time, RNA is transcribed and protein translated until the plasmid DNA is lost after a few cell divisions. This expression of the plasmid DNA, mRNA, and protein is transient (temporary).In some cases, the plasmid DNA is integrated into the host cell genome. This is usually accompanied by forced expression using a selection antibiotic and sometimes a cloning step (to be sure all cells have the same integration site). Once the DNA is stable, the cell line can be frozen and used to express protein for many years. Clones may even be screened for those expressing the highest amount of protein.
My question is not addressed here, how can I contact Technical Service for assistance?
Ask a Scientist here.
Articles
Transfection is the introduction of DNA, RNA, or proteins into eukaryotic cells and is used in research to study and modulate gene expression. Thus, transfection techniques and protocols serve as an analytical tool that facilitates the characterization of genetic functions, protein synthesis, cell growth and development.
This brief webinar provides an overview of what transfection is and the methods that are used to introduce DNA or RNA into eukaryotic cells.
Protocols
Calcium Phosphate Transfection Kit Protocol
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service