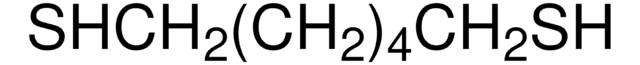381462
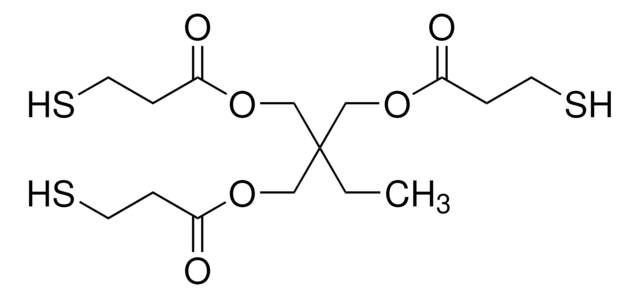
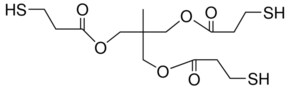
Pentaerythritol tetrakis(3-mercaptopropionate)
>95%
Recommended Products
Quality Level
Assay
>95%
refractive index
n20/D 1.531 (lit.)
bp
275 °C/1 mmHg (lit.)
density
1.28 g/mL at 25 °C (lit.)
SMILES string
SCCC(=O)OCC(COC(=O)CCS)(COC(=O)CCS)COC(=O)CCS
InChI
1S/C17H28O8S4/c18-13(1-5-26)22-9-17(10-23-14(19)2-6-27,11-24-15(20)3-7-28)12-25-16(21)4-8-29/h26-29H,1-12H2
InChI key
JOBBTVPTPXRUBP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Polymeric degradable networks through thiol-ene click reactions with tri/tetra-acrylates.
- Thiol-ene-methacrylate composites, which are applicable as dental restorative materials.
- Network solid polymer electrolytes based on polydimethylsiloxane, for lithium-ion batteries.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Documents related to the products that you have purchased in the past have been gathered in the Document Library for your convenience.
Difficulty Finding Your Product Or Lot/Batch Number?
How to Find the Product Number
Product numbers are combined with Pack Sizes/Quantity when displayed on the website (example: T1503-25G). Please make sure you enter ONLY the product number in the Product Number field (example: T1503).
Example:
Additional examples:
705578-5MG-PW
PL860-CGA/SHF-1EA
MMYOMAG-74K-13
1000309185
enter as 1.000309185)
Having trouble? Feel free to contact Technical Service for assistance.
How to Find a Lot/Batch Number for COA
Lot and Batch Numbers can be found on a product's label following the words 'Lot' or 'Batch'.
Aldrich Products
For a lot number such as TO09019TO, enter it as 09019TO (without the first two letters 'TO').
For a lot number with a filling-code such as 05427ES-021, enter it as 05427ES (without the filling-code '-021').
For a lot number with a filling-code such as STBB0728K9, enter it as STBB0728 without the filling-code 'K9'.
Not Finding What You Are Looking For?
In some cases, a COA may not be available online. If your search was unable to find the COA you can request one.
Articles
The Progress in Development of Dental Restorative Materials
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service