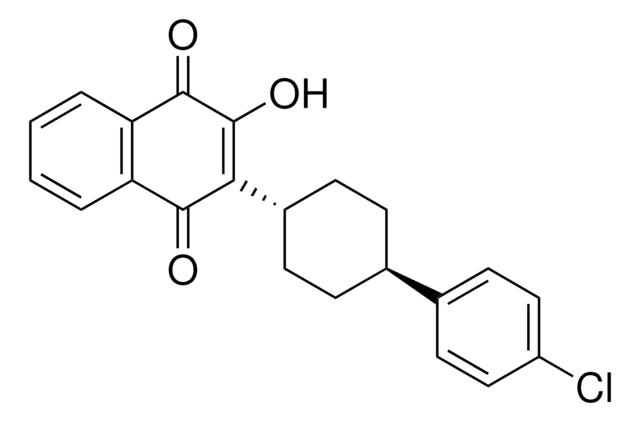
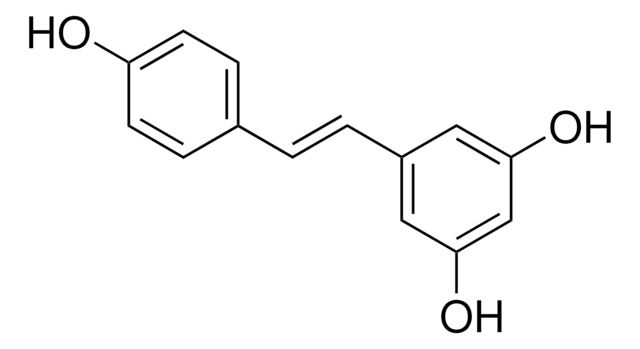
Recommended Products
Assay
98%
form
solid
optical activity
[α]20/D +76°, c = 0.5 in methanol
mp
156-157 °C (lit.)
antibiotic activity spectrum
parasites
viruses
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
Mode of action
enzyme | inhibits
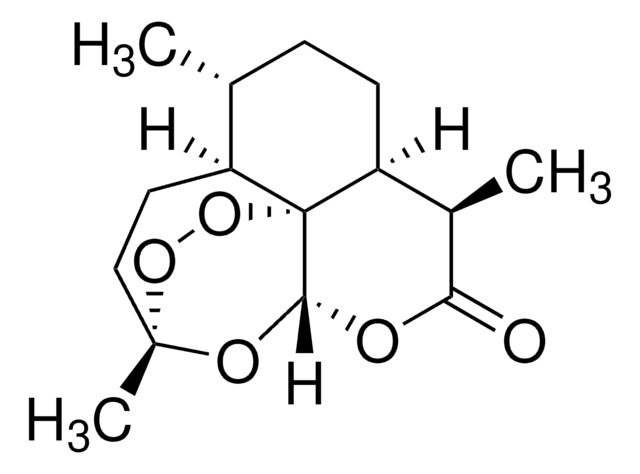
SMILES string
C[C@@H]1CC[C@H]2[C@@H](C)C(=O)O[C@@H]3OC4(C)CC[C@@H]1[C@@]23OO4
InChI
1S/C15H22O5/c1-8-4-5-11-9(2)12(16)17-13-15(11)10(8)6-7-14(3,18-13)19-20-15/h8-11,13H,4-7H2,1-3H3/t8-,9-,10+,11+,13-,14-,15-/m1/s1
InChI key
BLUAFEHZUWYNDE-NNWCWBAJSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- in drug susceptibility assays
- as a standard for its quantification from artemisinin from hairy roots
- to check cell viability using 3-(4, 5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
- as an antimalarial agent to investigate the role of autophagy-related (ATG) genes throughout the P. falciparum asexual and sexual blood stages
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Documents related to the products that you have purchased in the past have been gathered in the Document Library for your convenience.
Difficulty Finding Your Product Or Lot/Batch Number?
How to Find the Product Number
Product numbers are combined with Pack Sizes/Quantity when displayed on the website (example: T1503-25G). Please make sure you enter ONLY the product number in the Product Number field (example: T1503).
Example:
Additional examples:
705578-5MG-PW
PL860-CGA/SHF-1EA
MMYOMAG-74K-13
1000309185
enter as 1.000309185)
Having trouble? Feel free to contact Technical Service for assistance.
How to Find a Lot/Batch Number for COA
Lot and Batch Numbers can be found on a product's label following the words 'Lot' or 'Batch'.
Aldrich Products
For a lot number such as TO09019TO, enter it as 09019TO (without the first two letters 'TO').
For a lot number with a filling-code such as 05427ES-021, enter it as 05427ES (without the filling-code '-021').
For a lot number with a filling-code such as STBB0728K9, enter it as STBB0728 without the filling-code 'K9'.
Not Finding What You Are Looking For?
In some cases, a COA may not be available online. If your search was unable to find the COA you can request one.
Which document(s) contains shelf-life or expiration date information for a given product?
If available for a given product, the recommended re-test date or the expiration date can be found on the Certificate of Analysis.
How do I get lot-specific information or a Certificate of Analysis?
The lot specific COA document can be found by entering the lot number above under the "Documents" section.
How do I find price and availability?
There are several ways to find pricing and availability for our products. Once you log onto our website, you will find the price and availability displayed on the product detail page. You can contact any of our Customer Sales and Service offices to receive a quote. USA customers: 1-800-325-3010 or view local office numbers.
What is the Department of Transportation shipping information for this product?
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
In what solvents is Product 361593, Artemisinin, soluble?
According to the chemicals encyclopedia published by the Royal Society of Chemistry, artemisinin is expected to be soluble in most aprotic solvents and slightly soluble in oil. Sigma-Aldrich tests the solubility of this product in chloroform at 25 mg/mL. Our quality control is also able to prepare a 0.5% solution in methanol in order to measure the optical rotation.
My question is not addressed here, how can I contact Technical Service for assistance?
Ask a Scientist here.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service