Single-Use Assemblies
Whether you are looking to introduce single-use manufacturing components into your current process or investigating how you can implement a single-use process train, Mobius® 2D and 3D single-use assemblies and solutions can help you meet your changing manufacturing requirements.
Mobius® 2D and 3D bag assemblies
Our Mobius® 2D and 3D assemblies provide efficient and scalable bioprocessing fluid management solutions, from media and buffer preparation to final fill applications. With a full spectrum of solutions to choose from, at varying levels of certification, you get greater flexibility, mobility, and support for your single-use technologies. Our assemblies and solutions are designed to reduce operator error and process risks while improving efficiency. With our proprietary EZ Fold technique, filling and deployment of our Mobius® 3D bag assemblies are made easy for operators.
We're committed to developing high-performance products that continually evolve with your needs. To ensure you stay ahead in your production/manufacturing, we provide standard ready-to ship assemblies that are off-the-shelf for immediate process needs.
Solutions include:
|
|
Scalable Single-use Assemblies to Meet Your Bioprocess Needs
Mobius® Essential Assemblies are standard, off-the-shelf, simple bioprocess bag and tubing assemblies that are ideally suited for all your non-critical applications. These plug-and-play assemblies are made of our proven PureFlex™ film and are available in both 2D bags (1 L - 50 L), and 3D bioprocess bags (100 L – 200 L), as well as bioprocess tubing assemblies.
These standard assemblies are easily integrated into your current processes providing you with a straight path for scale up to our Mobius® MyWay assemblies with PureFlex™ film.
- Eliminate design and manufacturing lead time
- 1 L - 200 L sizes that can easily scale up to premium assemblies under Mobius® MyWay program
- Ready-to-download Emprove® Dossier with comprehensive product and regulatory information
Simply select your non-critical bioprocess bag and tubing assemblies, click to cart today.
Products
Mobius® Assembly Certification and Release Testing
Mobius® assemblies are available in several levels of certification. Certification of an assembly is based on the qualification of the components in the assembly, the level of leak testing performed during manufacturing, and the testing performed on the assembly lot after manufacturing. The certification level also impacts the shelf life and sterility claims that are made for the assembly.
Helium Integrity Manufacturing Release Test for Increased Integrity Assurance
Mobius® single-use assemblies* can also be tested with a Helium Integrity Manufacturing Release Test. Our Helium Integrity Test is validated to detect defects as small as 2 µm, and is designed to test the entire single-use assembly, including tubing and connection points. Incorporating the Helium Integrity Test into your integrity assurance strategy reduces the risk of leaks or microbial ingress in your manufacturing process.
* Mobius® assemblies for Helium Integrity Test is subject to the design space rules. Please find the details in the Helium Test Data Sheet.
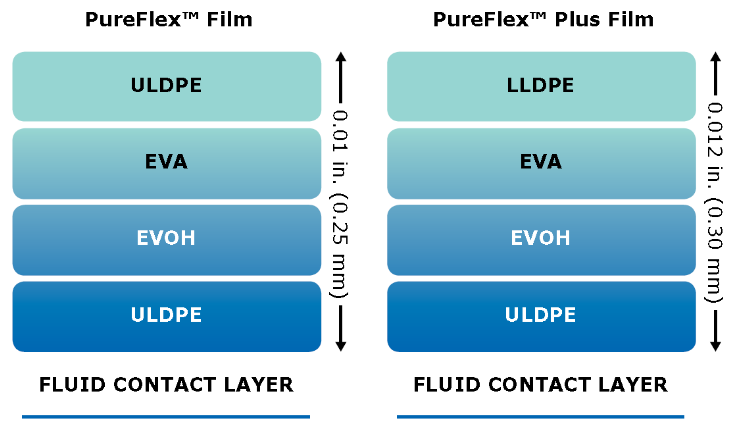
Single-use film technology
Our PureFlex™ single-use process container films are used for the construction of Mobius® single-use 2D and 3D bag assemblies. Both PureFlex™ and PureFlex™ Plus films consist of a single fluid contact layer with a low extractable profile, chemically resistant materials, and zero animal-derived components, offering the flexibility and robustness you need throughout your single-use manufacturing processes.
Emprove® Program and dossiers
The Emprove® Program supports your risk assessment and assists in developing more robust processes. It provides comprehensive and thorough documentation of our filters and single-use components, as well as pharma raw and starting materials. It not only covers the latest regulatory requirements, but also anticipates industry expectations not yet covered by regulation. The Emprove® Program is organized in three different types of dossiers. Every dossier supports you throughout different stages of your operations: material qualification, risk assessment, and process optimization.
Quality control and assurance to support your bioprocess risk mitigation
With a global network of sales development specialists and engineers we can support you with system demonstration, design and implementation, at-scale process simulation, and feasibility studies, as well as validation studies and documentation, supported by BioReliance® validation services.
We take a holistic risk mitigation approach covering:
- Component and assembly qualification
- Bag and assembly manufacturing process
- Integrity testing
- Packaging, irradiation, and shipping
Related Product Resources
Product Specification
Brochure: Single-use Fluid Management: 2D and 3D Assemblies, Filtration, Storage, and Transportation Solutions
Tech Brief: Holistic Buffer Preparation
Data Sheet: Mobius® 2D Freeze Assembly
Spec Sheet: Mobius® Essential Assembly
Extractables and Leachables
Webinar: Process Equipment Characterization – How Standardized Extractables Data Support E&L Risk Assessment
White Paper: The Role of BPOG Extractables Data in the Effective Adoption of Single-use Systems
White Paper: PureFlex™ and PureFlex™ Plus Disposable Process Container Films Extractables Evaluation
Poster: PureFlex™ Plus Single-use Process Container Film - Extractables and Leachables Study
Webinar: The Role of BPOG Extractables Data in the Effective Adoption of Single-use Systems
To continue reading please sign in or create an account.
Don't Have An Account?