Ficoll® 400 for Gradient Centrifugation
Product No. F4375
CAS #: 26873-85-8
Ficoll® 400 is a highly branched polymer formed by the copolymerization of sucrose and epichlorohydrin. Ficoll® 400 is completely non-ionic. Because of the abundance of hydroxyl groups, Ficoll® 400 is very hydrophilic and extremely water-soluble. The most common application for Ficoll® 400 is as a density gradient medium for the separation and isolation of eukaryotic cells, organelles and bacterial cells. Density ranges up to 1.2 g/ml can be attained. It has also been utilized in a variety of other applications.
Physical Properties
Appearance: White to white with faint yellow cast powder
Loss on drying: Not more than 5%1
Molecular Weight: 400,000 +/- 100,000 as determined by intrinsic viscosity1
Specific Rotation: +56.5° at 20 °C (C=1% in water)1
Intrinsic viscosity: approximately 0.17 dl/g1
Dialyzable material including NaCl: less than 1%1
Stokes Radius: approximately 10 nm1
Unlike sucrose, solutions of Ficoll have relatively low osmolality. Despite this, Ficoll's density in aqueous solutions is comparable to that of sucrose.
Because of the high molecular weight and low content of dialyzable material, Ficoll has a much lower permeability towards cell membranes than sucrose. Therefore, cells can be expected to collect at a lower density in Ficoll gradients than in sucrose gradients. Because of its low membrane permeability and low osmotic pressure, separations in Ficoll normally result in better preservation of cell function and morphology.
Storage/Stability
Stored properly as a powder at room temperature Ficoll 400 can be expected to have a shelf-life of 5 years.
Solubility /Solution Stability
Concentrations of 50% (w/v) can be attained in water. Ficoll should be added slowly with constant stirring.
We test the solubility of Ficoll 400 at 1g in 10mL of deionized water yielding a clear to slightly hazy, colorless to faint yellow solution.
Ficoll is stable in alkaline and neutral solutions. At pH values below 3, it is rapidly hydrolyzed, particularly at elevated temperatures.
Ficoll can be sterilized by autoclaving at a neutral pH, at 110 °C for 30 minutes.
Strong oxidizing and reducing agents are to be avoided.
Procedure
Centrifugation
Ficoll® 400 can be used for gradient centrifugation in all types of centrifuge rotors and for separation at unit gravity. For centrifugation, both discontinuous and continuous gradients are possible. Discontinuous gradients offer two main advantages: First, the abrupt changes in Ficoll 400 density mean that isolated cells are found in sharp bands at the interface between layers of different densities. This allows for easy removal of the sample with a pipette. Second, cells with great differences in density can be easily isolated with as few as two density layers. This is achieved by choosing densities that will prevent one or more cell types from entering the lower phase, banding these cell types at the interface.
To estimate the densities required for a particular application, consult the table below:
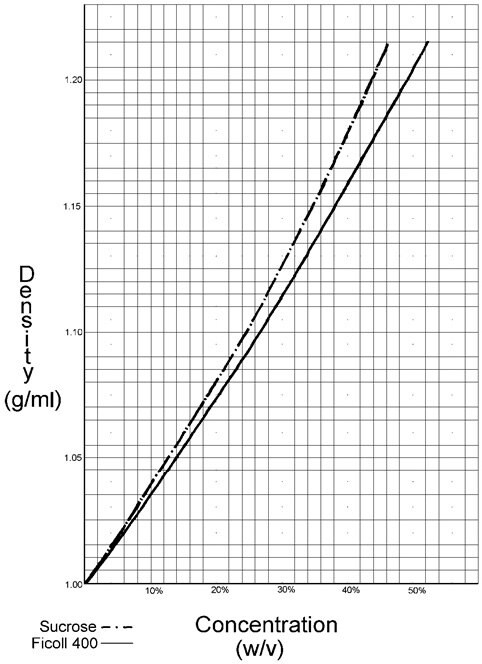
Figure 1.The graph of densities of Ficoll as a function of concentration follows.
Preparing a discontinuous gradient:
- Prepare Ficoll 400 in buffer or isotonic sucrose solution (0.25M) at concentrations that should separate the material of interest. Most cells and organelles have a buoyant density between 1.0 and 1.2 g/ml in Ficoll 400. Often a two-layer gradient is sufficient. Solutions made at this step may be stored in the refrigerator, but should be used at room temperature.
- In standard centrifuge tubes, make layers
(approximately 1 cm deep) with the densest layer on the bottom.
- Taking care not to mix the layers of the gradient, carefully layer the sample on top. Stir the sample and the uppermost Ficoll 400 layer gently with a glass rod to eliminate the interface before centrifugation.
During centrifugation the various particles will collect either in or between the Ficoll layers, depending on their density. Upon completion of centrifugation, pipette off the various phases and remove the Ficoll from the fractions of interest. Ficoll may be removed from isolated cells and organelles by repeated cycles of dilution with buffer followed by centrifugation. Residual amounts of Ficoll 400 in the sample can be estimated with the anthrone reaction.2
In some instances, a continuous or linear density gradient may be desired. This can be easily prepared using a gradient mixer. For simple separations, a homologous Ficoll 400 solution without a gradient can be used. Fractionation is accomplished by stepwise increases in centrifugation speed. Ficoll has also been employed in zonal centrifugation studies.3
Unit gravity sedimentation through a density gradient is widely used to separate cells that are sensitive to centrifugation. Cells with similar densities but different sizes can also be efficiently separated at unit gravity.4,5,6
Ficoll-Hypaque Density Media (Histopaque)
Applications calling for Ficoll-Hypaque employ a mixture of Ficoll and sodium diatrizoate. We offer preformed mixtures of three different densities under the product names Histopaque-1077, 1083, and 1119.
Nucleic Acid Hybridization
Ficoll 400 is a constituent of Denhardt’s solution used in Northern and Southern blot analysis. Ficoll reduces non-specific binding of material to nitrocellulose membranes during nucleic acid hybridization.7
We offer a Denhardt’s solution (Product Number D2532), 50X concentrate, tested for use in nucleic acid hybridization. Typical hybridization solutions require a 5X concentration of Denhardt's solution.
Immunological Applications
Ficoll 400 has been employed as a hapten carrier, and has been conjugated to dinitrophenol, trinitrophenol, and fluoresceinisothiocyanate for the purpose of enhancing primary immune response in mice. Conjugates with a range of substitution levels and minimal toxicity are easily prepared.8,9
Chemically Defined Cell Culture Media
Ficoll is used with and without serum-derived growth factors to support the growth of both primary cultures and established cell lines.10,11
Concentration Dialysis
Ficoll 400 is useful for concentrating solutions by dialysis, since its high molecular weight prevents it from crossing the dialysis membrane. Osmotic pressure draws water across the membrane into the solution of Ficoll 400, effectively concentrating sensitive materials.1
Electrophoresis
Continuous flow electrophoresis usually requires a stabilizer in the electrolyte. Ficoll 400 is often used for this application.12,13
Phase Partitioning
Phase partitioning separates cells on the basis of surface properties. Ficoll 400 is combined with polyethylene glycol in two-phase systems, and with dextran and polyethylene glycol in three-phase systems.14,15
Physiological Perfusion and Cell Stabilization Solutions
Ficoll has been added to physiological saline perfusate during monitoring of protein excretion in vessels. Vitrified mouse embryos have been diluted with solutions containing 30% Ficoll plus 0.5 M sucrose.17 Isolated rat kidneys were perfused with Tyrode’s solution containing 4.7% Ficoll 400.18
*Ficoll is a registered trademark of Pharmacia. Information on physical properties and applications was obtained from Pharmacia.
References
To continue reading please sign in or create an account.
Don't Have An Account?