This is one of the most critical stages during the preparation of membrane proteins. During the solubilization stage, membrane proteins are extracted from their natural environment, the lipid membrane, to an aqueous environment by the use of detergents. Detergents act by disintegrating the lipid bilayer while incorporating lipids and proteins in detergent micelles.
The hydrophobic surface areas of the membrane proteins and the lipid “tails” are buried in the hydrophobic interior of the detergent micellar structures, while hydrophilic parts of the proteins are in contact with the aqueous environment (Fig 1.1). An efficient solubilization dissociates most lipid-protein and protein-protein interactions, thereby allowing the separation of proteins.
The target protein can be purified in the presence of detergent by applying essentially any of the existing protein purification techniques available for soluble proteins. A successful solubilization protocol extracts the membrane protein at a high yield and results in stable protein-detergent complexes (or protein-lipid-detergent complexes) where the protein retains its active conformation.
Some membrane proteins require the interaction with native lipids from the lipid bilayer or added exogenous lipids to remain in their active conformation. In such cases, it is essential that the solubilization protocol enables the formation of a stable protein-lipid-detergent complex and that it does not remove the required native lipid(s) associated with the target protein. Harsh solubilization and purification procedures may lead to the removal of such essential lipids, and hence inactivation of the protein.
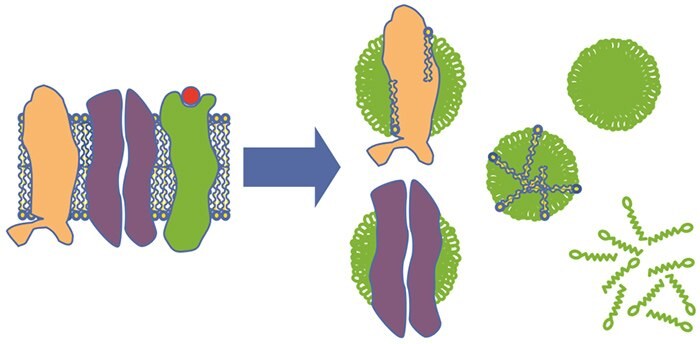
Figure 1.1.Schematic drawing of detergent solubilization of membrane proteins. Membrane proteins are transferred from the natural lipid bilayer (blue and yellow) to complexes with detergent (green) and, in some cases, lipids. A lipid-detergent micelle, a detergent micelle, and free detergent are also shown.
Detergents and CMC
Detergents are amphipathic substances with a polar (hydrophilic) head group and a nonpolar, (hydrophobic) tail. The polar part can be nonionic, anionic, cationic, or zwitterionic and detergents are often classified accordingly (i.e., a detergent with an anionic polar part is referred to as an anionic detergent). Advantages and disadvantages of the different classes of detergents are listed in
Table 1.3.
Above a certain concentration in an aqueous environment, detergent molecules associate to form multimolecular complexes, micelles, with hydrophobic interiors and hydrophilic surfaces. This concentration is referred to as the critical micellar concentration (CMC). The CMC is different for different detergents and it also varies with pH, temperature and ionic strength.
In general, all buffers and solutions used for membrane protein preparations (for solubilization, purification, storage, etc.) should have a detergent concentration above the CMC.
Several detergents that are recommended for solubilization of membrane proteins are listed in Table 1.4. Chemical structures of a few example detergents from the different classes are shown in Figure 1.4.
1N = nonionic; Z = zwitterionic
2At 20 °C to 25 °C and ~50 mM Na+
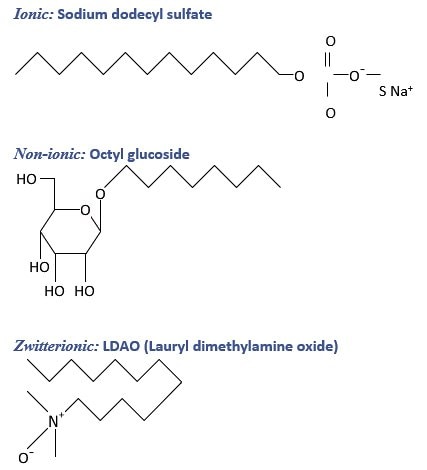
Figure 1.4.Chemical structures of selected detergents from the different classes.
Detergent screening
Several different detergents and conditions should be screened to establish the best conditions for each membrane protein. Solubilization yield is monitored by assaying for the target membrane protein in the solubilized fraction. In the protocol below, non-solubilized material is removed by ultracentrifugation.
It is also possible to apply non-clarified solubilisate directly to specially designed chromatography columns or multiwell plates that can handle cellular debris (Performing a small-scale expression screening of histidine-tagged membrane proteins from E. coli lysates). Filtration can also be used for clarification.
Protein detection may be performed by Western blot after SDS-PAGE. For low-expressed proteins, a chromatographic enrichment step may be required before protein detection is possible (Conditioning). For highly expressed proteins, solubilization yield can be estimated by SDS-PAGE with Coomassie Blue staining.
In addition to determination of yield and homogeneity, it is often necessary to monitor protein activity, which is the best indication of intact protein structure (and function). For some membrane proteins, functional assays can be applied to the detergent-solubilized protein. In other cases, reinsertion of the membrane protein into an artificial lipid bilayer (membrane protein reconstitution) is necessary to perform a functional assay.
General detergent screening procedure
Buffer preparation
PBS: 10 mM phosphate, 2.7 mM KCl, 137 mM NaCl, pH 7.4
Tris buffer: 20 mM at neutral pH Table 1.5. Additives to PBS or Tris buffer for detergent screening
For a list of recommended detergents, see Table 1.4.
Solubilization
- Starting with the membrane preparation of known protein concentration, combine materials as shown in Table 1.5 to give a total volume of 1 mL. Incubate with gentle mixing at the desired temperature for the time indicated.
- Centrifuge at 100 000 × g at 4 °C for 45 min.
- Assay the supernatant for target protein. Generic assays that can be used are SDS-PAGE (Performing a purity and homogeneity check), rapid affinity purification followed by gel filtration (Performing a purity and homogeneity check) and affinity tag specific assays (e.g., a dot blot using tag-specific antibodies). Also, assays for membrane protein activity should be considered.
The expression screening protocol in Performing a small-scale expression screening of histidine-tagged membrane proteins from E. coli lysates can be adapted to a combined solubilization screening and binding screening protocol.
The results from a preliminary solubilization screening of EM29, an Mr 29 000 histidine-tagged membrane protein from E. coli, is shown in Figure 1.5. A strong band at the expected position for the target protein indicates high expression and/or efficient solubilization. FC12, Triton X-100 and LDAO gave weaker bands than the other tested detergents. It was thus concluded that these detergents were less suitable for solubilization of this protein. It should be stated also that high or reasonable yields must be combined with preservation of activity and stability in detergent solution after solubilization—which is the next screening analysis that should be considered.
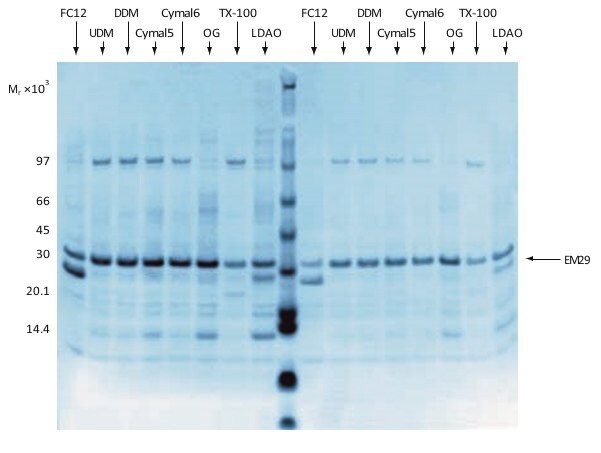
Figure 1.5.Analysis by SDS-PAGE and Coomassie Blue staining of a combined expression screening and preliminary solubilization screening of EM29. Eight different detergents were tested and elution was done in two steps (left and right half of the gel, respectively). FC12 = FOS-Choline 12; UDM = undecyl maltoside; DDM = dodceyl maltoside; OG = octyl glucoside; TX-100 = Triton X-100; LDAO = lauryl dimethylamine oxide. Data kindly provided by Dr. Said Eshaghi, Karolinska Insitute, Stockholm, Sweden.
Specific lipids may be associated with the protein in the native membrane, and their presence can be essential for protein activity. Retaining activity requires that these lipids are still present after solubilization and purification. Harsh solubilization and purification conditions can lead to removal of such lipids, resulting in inactivation of the membrane protein.
DDM is often a good detergent to try in initial solubilization tests.
CHAPS and digitonin have been reported to work particularly well for solubilization of membrane proteins from Pichia pastoris.
Solubilization screening for GST-tagged membrane proteins produced in E. coli
The method described below (Figure 1.6.) provides a simple and rapid method to select for optimal solubilization conditions to obtain the highest yield of GST-tagged membrane protein. This screening procedure is based on the affinity of GST for Glutathione Sepharose 4B media. The detergents selected for the screening must not affect the GST-binding activity.
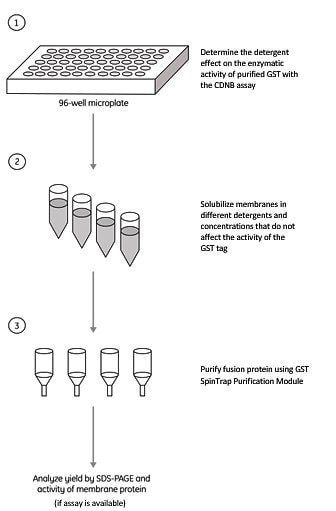
Figure 1.6.Detergent screening assay for a GST-tagged membrane protein.
Materials
GST Detection Module
GST SpinTrapTM Purification Module
GST MultiTrap 4B
Binding buffer: 10 mM phosphate, 2.7 mM KCl, 137 mM NaCl, pH 7.4 (PBS)
Elution buffer: Binding buffer supplemented with 0.2% (w/v) detergent and 10 mM reduced glutathione
Solubilization screening
If the enzymatic activity of GST (as an indicator of GST binding activity) in the presence of the selected detergents is unknown, evaluate GST activity in the detergents using the CDNB assay provided in the GST Detection Module. Compare GST activity in the presence and absence of each detergent. GST is known to be fully active in DDM, CHAPS, octyl glucoside, Tween 20, Triton X-100, Brij35 and NP 40 at detergent concentrations between 0.3 to 10 times CMC for each detergent.
- Disrupt cells by lysozyme treatment combined with freeze-thawing and isolate membranes by centrifugation at ~50,000 × g for 30 min at 4 °C (see “Membrane preparation from E. coli” in Performing a cell disruption and membrane preparation). Higher speed and longer centrifugation times (e.g., 100 000 × g for 60 min) may be required if more harsh cell disruption methods are used.
- Solubilize the membrane pellet (1:10 w/v) in 5% (w/v) detergent solutions for 2 h on ice with mild agitation (see “Detergent screening” further up for additional information).
- Clarify by centrifugation at ~50 000 × g for 30 min at 4 °C (or at 18 000 × g for 60 min at 4 °C).
- Decant 500 µL of the supernatant and purify with GST SpinTrap Purification Module or GST MultiTrap 4B according to the supplied instructions. Elute with 500 µL elution buffer. Assay for GST activity with GST Detection Module.
- Analyze by SDS-PAGE (see Performing a purity and homogeneity check).
Optimization of solubilization conditions
After establishing the initial conditions for solubilization, the conditions can be optimized by further screening with one or a limited number of detergents. Useful screening parameters to investigate are:
- ratio of protein concentration to detergent concentration. Protein concentrations in the range of 1 to 10 mg/ml are typical
- solubilization time and mixing conditions (e.g., mild agitation, end-over-end rotation, or vigorous stirring)
- pH
- ionic strength
Size homogeneity can be used as an indicator of stability (and therefore optimum solubilization conditions) because membrane proteins often oligomerize or aggregate rapidly when destabilized. Size homogeneity can be rapidly evaluated using SuperdexTM 200 5/150 GL (see “Size homogeneity characterization” in Performing a purity and homogeneity check). Figure 1.7 demonstrates how SuperdexTM 200 5/150 GL (column volume 3 mL) can be used to screen for homogeneity under various pH and salt conditions.
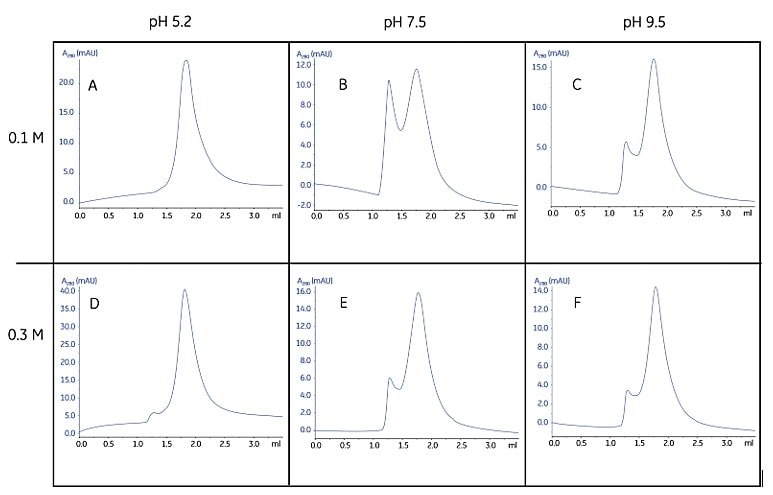
Figure 1.7.Screening of pH and ion strength conditions for optimal homogeneity and stability of a detergent-protein complex. Rapid gel filtration with Superdex 200 5/150 GL showed a symmetrical peak when the separation was performed at pH 5.2 in 0.1 M NaCl (A), indicating a homogenous protein under these conditions. At somewhat higher salt concentration (D) a small peak appeared close to the void volume, indicating that oligomerization or aggregation appeared to a limited extent. At both pH 7.5 and pH 9.5 significant peaks were obtained close to the void volume, indicating severe oligomerization or aggregation. The complete screening procedure was achieved in only a few hours, including the time for column equilibration. Sample consumption was 6 × 10 µL for the complete screen. Data kindly provided by Dr. Said Eshagi, Karolinska Institute, Stockholm, Sweden.
Mixtures of detergents could also be considered.
Additives such as lipids and small amphiphiles (e.g., 1,2,3-heptanetriol) or known ligands for the target protein may be useful to test. Small amphiphiles may affect crystallization behavior by changing the size of detergent micelles. Ligands are believed to reduce the dynamics of the protein structure and thus stabilize the protein in detergent solution.
Solubilization for purification
After optimization, the solubilization protocol is performed at a scale that is appropriate for the amount of membrane protein that needs to be obtained.
It is recommended to proceed with purification immediately after solubilization, to minimize the loss of membrane protein activity due to aggregation, loss of structure, or proteolytic degradation.
A detergent that works well for the solubilization of a particular membrane protein may be less suited for other operations with the same protein (e.g., crystallization). Procedures for exchanging detergents are described in Conditioning.
Solubilization with organic solvents
Small and stable membrane proteins are sometimes possible to extract from the lipid bilayer in their active form by the use of organic solvents. For instance, a 110 amino acid membrane protein was extracted from E. coli with a chloroform:methanol mixture, and initial purification of the extract was done in this environment using an organic solvent resistant chromatography medium and column (Figure 1.8; 13). For this membrane protein, a 1:1 ratio of chloroform:methanol gave the best separation.
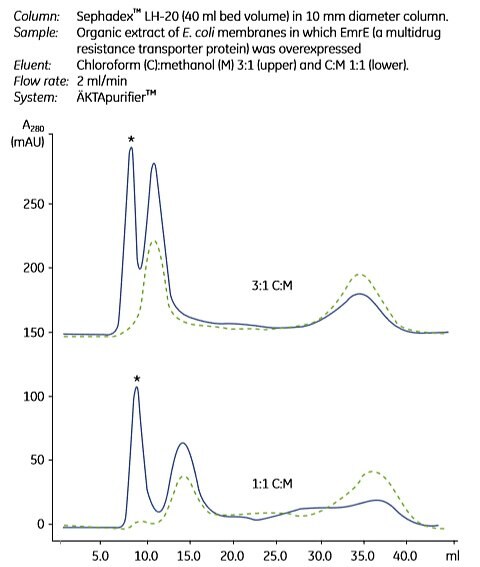
Figure 1.8.Gel filtration separation of solvent-extracted membrane proteins. Solid line = organic extract of E. coli membranes in which EmrE (a multidrug resistance transporter protein) was overexpressed; broken line = organic extract of “blank” E. coli membranes. Two ratios of chloroform (C) and methanol (M) were used. The asterisk marks EmrE containing peaks. Used with permission. Copyright 2002 Elsevier Science (USA) Publication.
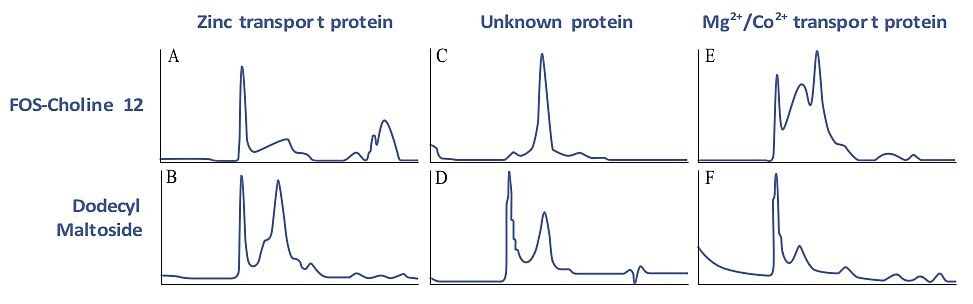
Figure 1.13.Size homogeneity characterization of membrane proteins by gel filtration. IMAC purifications of three E. coli membrane proteins were analyzed by gel filtration using Superdex 200. Each protein was purified separately by IMAC in the presence of two different detergents. Gel filtration analysis was done with the same detergent as used for purification. A symmetric peak (which is not at the void volume), as in chromatogram C, shows that the protein did not aggregate, indicating that purification was successful. Blue line = A280 trace. Used with permission. Copyright 2005 The Protein Society.
To continue reading please sign in or create an account.
Don't Have An Account?