Virus Concentration by Ultrafiltration
- Virus Isolation, Propagation, and Characterization
- General Methods for Viral Propagation
- Virus Concentration for High Titer Virus Stocks
- Virus Concentration with Amicon® Ultra Centrifugal Ultrafiltration Devices
- Conclusions
- Centrifugal Ultrafiltration Devices
Virus Isolation, Propagation, and Characterization
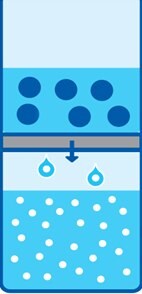
Figure 1.Ultrafiltration for concentration of virus. Fluid passes through the membrane filter, while large viral particles are retained.
Viruses can be propagated in cell culture to generate virus stocks. Cultured cells are inoculated with viral stock from seed virus, a commercial source, or infected tissue. After incubation, infected cells are lysed to harvest viral particles, or released virus is harvested directly from cell supernatants. The virus stock is then titered, aliquoted, and stored at −80 °C for later use.
Isolation of virus from clinical samples also exploits cell culture for viral propagation. Clinical samples are prepared by sample homogenization and extraction into a liquid medium, which is then centrifuged and used to inoculate cell cultures.
In order to achieve high titer virus stocks, it is necessary to concentrate purified virus particles. Amicon® Ultra and Centricon® Plus centrifugal ultrafiltration devices with regenerated cellulose membranes can be used to concentrate virions and virus solutions (Figure 1). This protocol describes the use of Amicon® and Centricon® centrifugal ultrafiltration devices for the concentration of three common types of viruses: lentivirus, adenovirus and adeno-associated virus (AAV).
General Method for Viral Propagation
- Packaging cells: Host cells were plated to achieve 80–85% confluence.
- Transfection: Cells were infected with viable virus particles.
- Harvesting virus: Depending on the virus, either cell supernatant or pelleted cells, or both, were used to purify the recombinant virions. Four rounds of freeze/thaw cycles were applied to release virus from cells. Cell debris was then separated from cell lysate by centrifugation.
- Virus purification: Virus was purified from cell lysate by density gradient ultracentrifugation or column/membrane chromatography.
- Purified virus solutions were concentrated and buffer exchanged into an appropriate storage solution using either passive dialysis, diafiltration, or centrifugal ultrafiltration with Amicon® Ultra and Centricon® Plus 70 devices.
Virus Concentration for High Titer Virus Stocks
To produce high titer viral stocks, the purified virus can be concentrated using centrifugal ultrafiltration. The correct choice of device, membrane material, molecular weight limit or cut-off, centrifugation speed, centrifugation time and buffer composition are critical for high recovery of infective viral particles. Amicon® Ultra centrifugal ultrafiltration units have specified nominal molecular weight limits (NMWL) defined by their filter membrane. Amicon® Ultra centrifugal ultrafiltration devices with a 50 kDa NMWL Ultracel® were used to concentrate adenovirus and adeno-associated virus (AAV) solutions. For lentivirus concentration, devices with both 50 kDa and 100 kDa NMWL membranes were used.
Factors to consider when selecting an ultrafiltration method and membrane nominal molecular weight size:
- Viral particle size: Size can be estimated from published sources, or by measurement techniques such as microscopy, laser diffraction, and dynamic light scattering.
- Size of key separation targets in solution: The size of proteins, antibodies, drugs, chemicals, and other particles that need to be separated will affect membrane size selection.
- Sample volume: Processing volumes ranging from ≤0.5 mL to 70 mL are compatible with centrifugal ultrafiltration (cUF) devices.
To retain viral particles, the molecular weight cut-off of the filter membrane needs to be smaller than the particle (~2 times smaller than the molecular weight of the viral particle), but large enough to allow smaller components to filter through
(Table 1).
Virus Concentration with Amicon® Ultra Centrifugal Ultrafiltration Devices
Table 2 shows results for concentration of adenovirus, lentivirus, and AAV from both crude cell lysate and virus purified by chromatography. Viral particles were effectively concentrated to high titers with high recoveries using Amicon® Ultra centrifugal ultrafiltration devices.
It is important to remember that most of the viruses cannot be efficiently concentrated and stored in low salt buffers. Centrifugal concentration of the virus in < 250 mM salt solutions leads to virus aggregation and decreased infectivity.
Concentrated viral stock can be filter-sterilized through 0.22 µm filters. Ultrafree®-MC GV filter units can be used for volumes of < 0.5 mL.
Conclusions
We developed a method to validate ultrafiltration using Amicon® Ultra and Centricon® Plus centrifugal devices as a fast and cost-effective means for achieving high titers of viruses. This method can be used to concentrate viruses from cell culture supernatants, cell lysates, and other sources. After concentration, viral stock can be filter-sterilized using Ultrafree® filter units. For highest recovery of infective viral particles, it is critical to select the proper ultrafiltration device, membrane filter, molecular weight cut off (MWCO), centrifugation speed, and centrifugation time.
Centrifugal Ultrafiltration Devices
* registered for IVD use
To continue reading please sign in or create an account.
Don't Have An Account?