Primary Cancer Cell Media
Tumors consist of a heterogeneous mixture of multiple interacting cell types including non-tumorigenic and malignant phenotypes such as tumor cells, cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs) and stromal cells. Only a small subpopulation are cancer cells capable of driving progression and ultimately leading to malignancy. These malignant cells possess unlimited proliferation potential, self-renewal and resistance to cytotoxic drugs. The complexity of tumors makes the purification and characterization of the malignant subpopulation of cancer cells difficult.
In vivo cancer models, including patient derived xenographs (PDX models), have been developed for establishing tumor cultures in living animals since direct in vitro isolation was not effective. After several rounds of serial in vivo transplantation of tumor tissue in severely immune-compromised mice, the cells of the primary tumor eventually develop into a stable tumor cell population. PDX mouse models are believed to conserve the original tumor characteristics including heterogeneous histology, clinical biomolecular signatures, malignant phenotypes and genotypes, tumor architecture and vasculature. However, these techniques are often expensive, time-consuming, elaborate, variable and they induce major changes in the initial primary tumor cells caused by the serial selection process in animals.
Traditional in vitro cancer culture media lack specificity for malignant cells. These media predominately supports the proliferation of benign stromal cells and differentiated cells (non-tumorigenic), thus leading to a gradual loss of the original malignant cell population overtime. The PromoCell Primary Cancer Culture System (C-28081), consisting of Primary Cancer Cell Medium D-ACF (C-28080, C-39880) and the NCCD-Reagent (C-43080), was designed to be the first universally applicable and cost-effective solution for in vitro isolation of long-term primary tumor culture from patient tumor samples or patient derived xenografts (PDX). The selection process uses a proprietary coating NCCD-Reagent and an optimized animal-free cancer cell culture media. The system makes it possible to reliably deplete benign cells from the culture while supporting the maintenance of malignant cancer cells. The system can also be used for other applications such as enriching malignant subpopulation(s) in established cell lines or depleting stromal cells and other non-cancerous cells from established primary cancer cell cultures.

Cell Culture Protocol
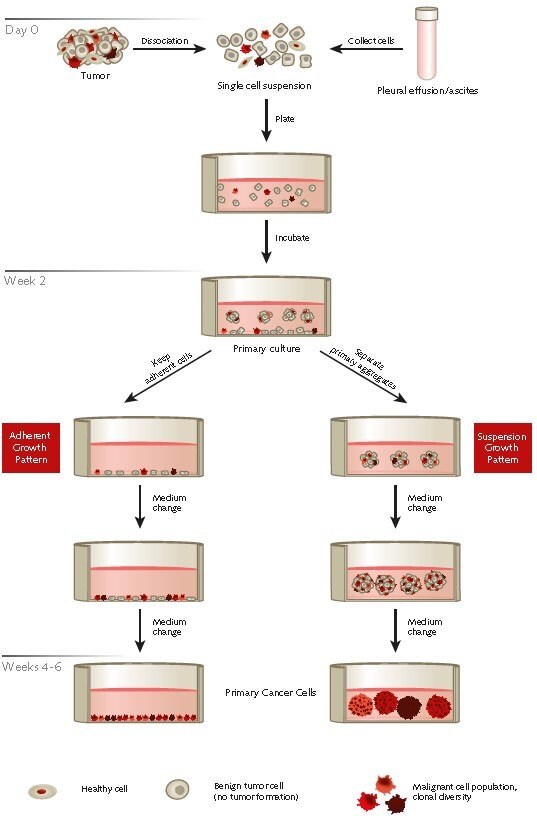
Figure 1. Protocol overview of the selective isolation of primary human cancer cells from patient derived tumor samples or effusions using PromoCell’s Primary Cancer Culture System.
A) Protocol for Establishment of Primary Cancer Cell Cultures
I. NCCD Treatment of the Plasticware (day 0)
The use of the NCCD-Reagent (C-43080) provided with the Primary Cancer Culture system is required for successful isolation and maintenance of cancer cells. Dilute the thawed NCCD-Reagent stock solution 1:20 with PBS. Use 100 μL/cm2 to treat the tissue culture vessel with the diluted NCCD-Reagent and leave the closed vessel for at least 1 hour at RT. Make sure that the NCDD covers the entire vessel surface. Aspirate the NCCD solution just before seeding the cells.
II. Tumor Cell Isolation Procedure (day 0)
- Wash and weigh the tumor tissue. Remove visible residues of healthy tissue from the tumor. Place the tumor sample in a tube and wash twice with a generous amount of PBS (D8537) and vigorous shaking. Weigh the tumor tissue in a pre-tared sterile petri dish.
- Homogenize the tumor tissue. Place the washed tumor sample on the lid of a petri dish. Add a small volume (1–2 mL) of Primary Cancer Cell Medium D-ACF (C-28081) to the tumor tissue and dissect it into small pieces using a scalpel. Homogenize the tissue to a “slurry” or into small pieces of roughly 1 mm3 by additionally mincing the tissue chunks using the scalpel.
- Wash the homogenized tumor tissue. Transfer the homogenized tumor tissue to a 50 mL tube using forceps. Add 10x the volume (w/v) of PBS (D8537) and vortex or mix vigorously. Let the tissue pieces settle for 2 minutes and then aspirate the supernatant.
- Perform the enzymatic digest of the tumor tissue. Resuspend the tissue pellet in Accumax solution (A7089) at a concentration of 20 mL/gram of tumor tissue. Incubate at RT with gentle but constant mixing. Digest until the solution becomes distinctly turbid. Depending on the type of tissue, this is typically the case after approximately 30–60 minutes.
- Remove tissue residues from the sample. Let the remaining tissue pieces settle down for 2 min. In order to obtain a single cell suspension, progressively filter the turbid supernatant using cell strainers of descending pore size down to 40 μM. Note: Discard the remaining tissue pieces.
- Dilute the sample with medium. Dilute the single-cell suspension at least 1:1 with Primary Cancer Cell Medium D-ACF (C-28081). Use a higher dilution ratio if the solution is still viscous.
- Obtain isolated single cells. Pellet the cell suspension for 10 min at 240 x g at RT and carefully aspirate the supernatant without disturbing the cell pellet.
- Determine the number of viable nucleated cells. Resuspend the cell pellet in 5 mL of Primary Cancer Cell Medium D-ACF (C-28081). Determine the number of viable nucleated cells using an appropriate method.
- Wash the cells. Pellet the cell suspension for 10 min at 240 x g at RT and carefully aspirate the supernatant without disturbing the cell pellet. Finally, resuspend the cell pellet in 1 mL of Primary Cancer Cell Medium D-ACF (C-28081).
- Plate the cells. Plate 100K-200K viable nucleated cells per cm2 in the prepared NCCD-treated tissue culture vessel(s). Plate 2.5–5 million nucleated viable cells per T-25 flask using 5 mL of medium. Plate 7.5 – 15 million nucleated viable cells per T-75 flask using 10 mL of medium. Add 50 μg/mL of Gentamicin (G1397) to the final volume and incubate at 37 °C with 5% CO2.
III. Primary Cancer Cell Culture
- Initiate of the primary tumor cell culture (day 0). Incubate the culture for a total of 10 – 14 days to let the primary tumor cell culture begin, but proceed with step two on day 6 after plating.
- Add fresh medium (day 6). On days 5 – 7, add an additional volume of the initial culture volume of fresh Primary Cancer Cell Medium D-ACF (C-28081) (w/o antibiotics) to the cells. Do not change the medium; simply add more fresh medium. Continue incubation until the culture reaches the stage described in step 3.
- Initiate a separate secondary suspension culture (days 10 – 14). The primary culture is ready for step 3 as soon as sufficiently large floating multicellular aggregates (i.e. ≥70 μM in diameter) have developed or small aggregates of fewer than 10 cells appear. Depending on the suspension cell pattern of your primary culture, continue with step 3a or 3b, whatever is more appropriate.
3a) Separation of larger aggregates (≥70 μM or ≥10 cells). To separate the established primary tumor cell aggregates, collect the used medium (containing the aggregates) in a separate 15 mL conical tube. Wash the remaining adherent cells twice with PBS (D8537), immediately add an appropriate amount of fresh Primary Cancer Cell Medium D-ACF medium (C-28081) and put them back into the incubator. Let the tube with the suspension aggregates stand upright for 15 minutes at RT to allow gravity sedimentation of the primary aggregates. Then gently aspirate the supernatant, leaving back 0.5 – 1 mL. Plate the sedimented primary aggregates with an appropriate amount of fresh Primary Cancer Cell Medium D-ACF medium (C-28081) in a separate new culture vessel treated with the NCCD-Reagent.
3b) Separation of small aggregates and single suspension cells. Collect the used medium containing the small aggregates/single suspension cells in a separate 15 mL conical tube. Wash remaining adherent cells twice with PBS (D8537), immediately add an appropriate amount of fresh fresh Primary Cancer Cell Medium D-ACF medium (C-28081) and return them to the incubator. Centrifuge the suspension cell sample for 10 minutes at 240 x g at RT. Leave 200 μL of the supernatant behind while gently aspirating the spent medium, since the pellet may be quite loose. Resuspend and plate the cells in an appropriate amount of fresh fresh Primary Cancer Cell Medium D-ACF medium (C-28081) in a separate new culture vessel treated with the NCCD- Reagent.
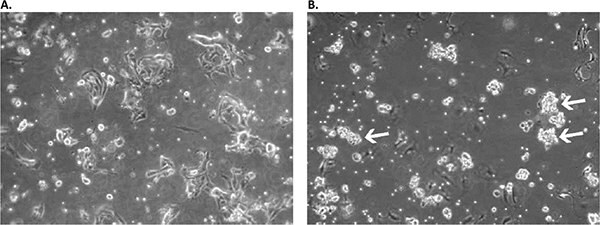
Figure 2. Appearance of primary culture of lung squamous cell carcinoma cells in the Primary Cancer Cell Medium D-ACF in early stages.A) Culture on day 2 after initial plating: a mix of residual erythrocytes, fibroblast- and epithelial-like adherent cells as well as floating single suspension cells can be observed. B) On day 11, the formation of floating multicellular cell aggregates is already prominent (white arrows). The culture was used for primary aggregate separation on day 13 and was additionally cultured in a new flask parallel to the original sample containing the remaining adherent cell fraction.
- Change medium during cancer cell selection (every 10 – 14 days). After successfully completing step 3, completely replace the medium of all samples every 10 – 14 days as described below. The appropriate media change technique will depend on the growth pattern of the corresponding primary isolate.
4a) Change medium for adherent cultures. Aspirate the used medium of adherent cells, wash the culture twice with PBS (D8537) and add an appropriate amount of fresh Primary Cancer Cell Medium D-ACF medium (C-28081) to the cells.
4b) Change medium for large cell aggregates (≥ 70 μM). Collect the used medium containing the suspension aggregates in a separate 15 mL conical tube. If applicable, wash remaining adherent cells twice with PBS (D8537), immediately add an appropriate amount of fresh Primary Cancer Cell Medium D-ACF medium (C-28081) and return them to the incubator. Let the tube with the suspension aggregates stand upright for 12 minutes at RT for gravity sedimentation of larger cell aggregates. Then gently aspirate the supernatant while leaving 0.5–1 mL behind. Carefully transfer the sedimented aggregates with a serological pipet into a NCCD-treated culture vessel containing an appropriate amount of fresh Primary Cancer Cell Medium D-ACF medium (C-28081).
4c) Change medium for small aggregates and single suspension cells. Collect the used medium containing the small aggregates/single suspension cells in a separate 15 mL conical tube. If applicable, wash remaining adherent cells twice with PBS (D8537), immediately add an appropriate amount of fresh Primary Cancer Cell Medium D-ACF medium (C-28081) and return them to the incubator. Centrifuge the suspension cell sample for 10 minutes at 240 x g at RT. Leave 200 μL of the supernatant behind when gently aspirating the spent medium, since the pellet may be quite loose. Resuspend the pellet in fresh Primary Cancer Cell Medium D-ACF medium (C-28081) and use a serological pipette to transfer the cell suspension into a NCCD-treated culture vessel containing an appropriate amount of fresh Primary Cancer Cell Medium D-ACF medium (C-28081).
- Determine the growth pattern of the isolated cancer cells (weeks 2 – 4). Adherently growing cancer cells can typically be identified within 2 – 4 weeks after plating, since they are present as slow-growing colonies. Follow the regular medium changing schedule for adherent cells without passaging the cells until they reach an adequate confluency level (see step 7).
- Use the purified malignant cells for your experiments (week 4+). After making sure that all unwanted benign cells have been eliminated, you can set up your experiments with the isolated cancer cells. Alternatively, the cellsmay be passaged and expanded further (see step 7).
- Passage the tumor cell primary culture. Passaging the cells before they proliferate to a high confluence level is not recommended. Until they do, continue changing the medium as described for step 4.
7a) Passage the suspension cultures. Increase the total culture volume by adding fresh medium and split it in two (or more) fresh NCCD-treated vessels. Use gravity sedimentation for the regular medium changes to reduce the amount of debris in the culture. Note: In contrast to other types of sphere cultures, it is not necessary to disaggregate cell clusters because aggregates of malignant cells propagate autonomously under these culture conditions.
7b) Passage the adherent cells. Prepare new NCCD-treated culture vessels. Depending on the overall confluence, perform a 1:1 or 1:2 split of the culture using Accutase (A6964).
B) Protocol for Depleting Contaminating Non-Cancerous Cells in Established Primary Cancer Cell Cultures
- NCCD-treatment of plasticware with TC surface. Dilute the thawed NCCD-Reagent stock solution 1:20 with PBS w/o Ca2+/Mg2+ (D8537). Use 100 μL/cm2 of culture surface to treat the tissue culture vessel with the diluted NCCD-Reagent and leave the vessel closed for at least 1 hour at RT. Make sure that the NCCD solution covers the entire vessel surface. Aspirate the NCCD solution just before seeding the cells.
- Determine the growth pattern of the malignant cells. Passage your established culture containing the malignant cells as usual. Plate a sample of the cells in a NCCD-treated vessel containing an appropriate amount of Primary Cancer Cell Medium D-ACF (C-28081). Grow the cells as described in protocol III. 1-7. Monitor the culture for proliferation of the malignant cells that interest you and determine their growth pattern.
Note: The malignant cells may grow adherently and/or as spheres in suspension. During the induction phase, they may proliferate more slowly than under your established standard culture conditions. However, the culture will recover as soon as the non-malignant cells have been substantially depleted (in passage 2 and 3) and the cultured cells have fully adapted to the new conditions.
- Clean up your culture. After you have identified the growth pattern of the malignant cells that interest you under these selective culture conditions, passage the culture into Primary Cancer Cell Medium D-ACF (C-28081). Expand and passage the cells as required ( III.7) for 2 to 3 times in order to deplete the culture of non-malignant cells.
Results
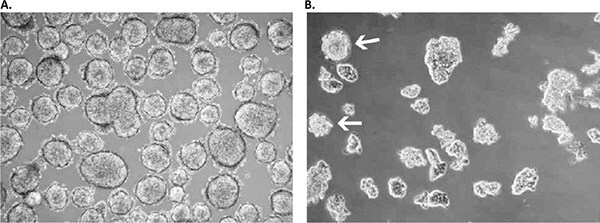
Figure 3. Primary culture derived from a low-grade small cell lung cancer with the Primary Cancer Culture System.A) The primary isolate was obtained after 4 weeks as a floating sphere-forming culture, which persisted in a near-quiescent state even after 6 months. B) Adding extra growth factors elicited significant expansion in the latent sphere culture with a doubling time of 3-4 weeks. Note that some spheres persisted under these modified culture conditions (white arrows), while the larger part of the culture proliferated as floating planar multicellular 2D sheets, which is a prototypical growth pattern for SCLC cells in vitro.
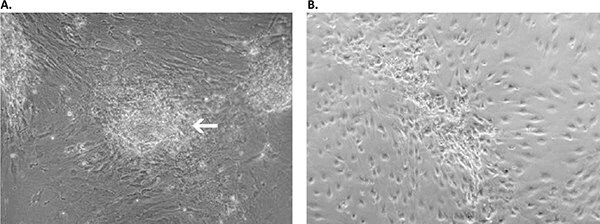
Figure 4. Primary culture derived from an invasive adenocarcinoma with the Primary Cancer Culture System. A) During the first two weeks of the isolation process, the cancer cells appeared as locally restricted, lightly-adherent convex cell clusters (white arrow) on top of the stroma layer. B) After 4 weeks, highly motile cancer cells began migrating from their original locations to cover the whole culture surface. These cells proliferated as a homogeneous population in the Primary Cancer Cell Medium D-ACF.
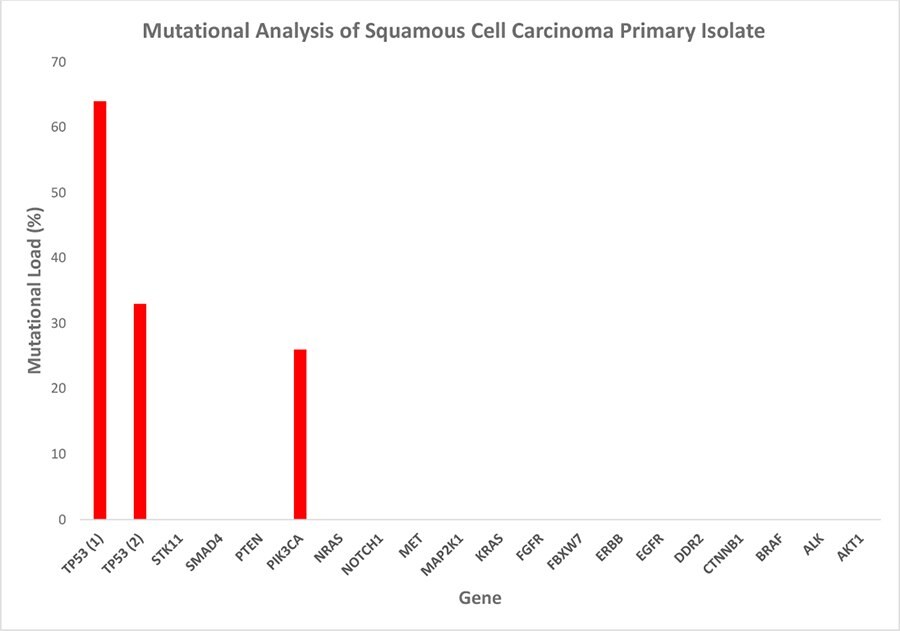
Figure 5. Mutational analysis of the squamous cell carcinoma primary isolate. The tumor panel test detected three hotspot mutations: one in the pik3ca gene and two in tp53. The high mutational load is indicative of a selectively enriched culture of malignant cells, while the differing percentages of the individual mutations suggest the maintenance of cancer cell subpopulation heterogeneity in vitro. Mutational load = percentage of mutated transcripts/total transcripts of the respective transcript variant.
References
To continue reading please sign in or create an account.
Don't Have An Account?