MCAT- 53™ Catalyst for Ruthenium Formation
A New Effective Ruthenium Formation Catalyst MCAT-53TM for C-H activated C-C coupling reactions in water that eliminates extra steps in C-C coupling and avoids organic solvents such as NMP
Modern catalytic functionalization reactions are continually advancing the ease of synthesis of valuable heterocyclic molecules; it is vital to ensure the environment is benign. Recently, Chicago Discovery Solutions developed MCAT-53™ as a recyclable, ligand-free ruthenium catalyst for C–H activation reactions and concomitant C–C bond formation in the presence of water.
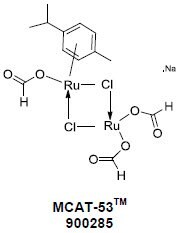
Ruthenium Formato catalyst MCAT-53 from Chicago Discovery Solutions LLC is tailor made for C-H activated C-C coupling reactions in water. There is no need to add acids, co-solvents, surfactants, oxidants or ligands and to perform additional steps for activation of the catalyst.Reactions using MCAT-53™ ameliorate the problems for undesirable toxicity and disposal costs2. After the reaction is complete and worked up, the aqueous medium can be reused (recycled) for another catalytic reaction.
Reactions using MCAT-53™ ameliorate the problems for undesirable toxicity and disposal costs2. After the reaction is complete and worked up, the aqueous medium can be reused (recycled) for another catalytic reaction.
Representative Application: synthesis of heterobiaryl compounds in water
Biaryl compounds are commonly prepared using well known aryl-aryl coupling reactions. Traditional aryl-coupling and C–H bonds activation methods (such as Suzuki-Miyaura and Stille cross-coupling reactions) are accomplished by tedious preparation of unstable, toxic organometallic reagents and the reactions are routinely run in harsh organic solvents (such as DMF, NMP, DMSO and toluene)1.Palladium-based catalysts, which often demand the use of phosphine-ligand systems, have dominated the realm of modern catalytic cross-coupling.
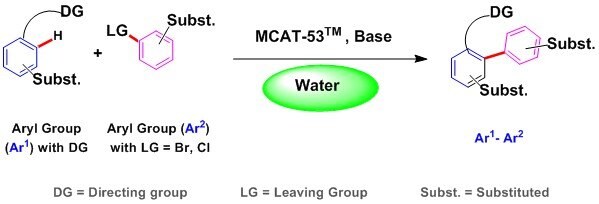
MCAT-53™ eliminates an extra step in synthesis by obviating the need to first convert leaving groups (such as halides) of the substrates into substituted derivatives (for example- boronic acid or ester)..
The catalyst is tailor made to work in plain water and there is no need to add acid, co-solvent, surfactant, or perform additional steps for activation for the catalyst. By simply combing an aryl halide with an aromatic coupling partner in the presence MCAT-53™ and a base (such as K2CO3), a safe and cost effective alternative to some of the very expensive Pd-catalysts and precatalysts3 is now available. Work up is simple as the product if solid may simply precipitate out and could be filtered off so avoiding use of any solvent to extract the product after the reaction was done in water as a solvent!
In a recent publication in Organic Research and Development, 2018ref5, MCAT-53 was demonstrated to work with following bromides, chlorides and heavily substituted halides:
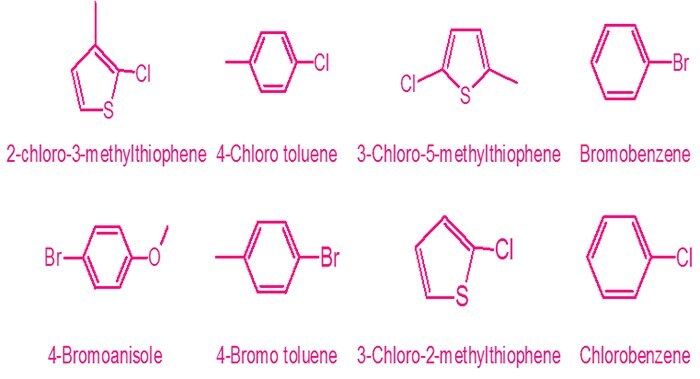
Bromides chlorides structures
A variety of pyridine derivatives can serve as excellent directing groups, and the reaction produces ortho-substituted products. Other nitrogen-based directing groups, including imines, oxime ethers, azobenzene derivatives, and nitrogen heterocycles (e.g., pyrazoles and isoxazolines) can be used5. Amides, which contain relatively basic oxygen atoms, could be used to direct these reactions.
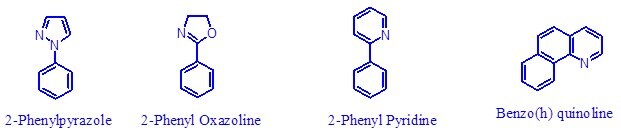
Phenylpyrazol
Publication Results:
- The successful scale up of C-H activated C-C coupling reactions to 12 mmole scale using MCAT-53 for C-H activated C-C coupling reaction.
- MCAT-53 catalyst loading reduction to barely 1 mole % for carrying out C-H activated C-C coupling reactions
- The scale up C-H activated C-C coupling reaction (with less loading) was really good as the product could be simply decanted off (the product was separated from water layer).
- The product if solid may simply precipitate out and could be filtered off so avoiding use of any solvent to extract the product after the reaction was done in water as a solvent!
- Manipulation of the mono and di ratios.
- Comparison to other catalyst showed that MCAT-53 is much superior to other Ru based catalyst in terms of improved isolated yields obtained by using MCAT-53.
- Use of the spent aqueous layer after the reaction completion (recycle- & re- use).
- The basis of structure determination of MCAT-53.
- Examples of reactions on many diverse substrates such as 2 phenyl pyridine, 2 phenyl pyrazole, and benzo (h) quinolone.
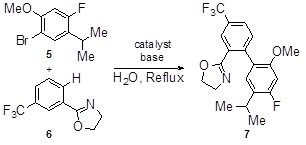
Synthesis of an advanced intermediate of CETP inhibitor, Anacetrapib, in water has been demonstrated to give a single regioisomer using the MCAT-53 catalyst5.
MCAT53 catalyst
The first of its class, bench and air stable, MCAT-53 will find utility in cost effective and greener alternatives in pharmaceutical and other chemical manufacturing processes.
Commercial availability of this catalyst and air stability with ease of transport and use opens the possibility of use of this new catalyst MCAT-53 to many other types of transformations.
References
To continue reading please sign in or create an account.
Don't Have An Account?