PEPPSI™ Catalyst - Introduction, Advantages and Applications
Professor Mike Organ, along with Drs. Chris O’Brien and Eric Kantchev, have developed an elegant Pd-NHC catalyst system. The title complex PEPPSI™ stands for Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation. The 3-chloropyridine functions as a ‘throw-away’ ligand, while the bulky IPr ligand improves the reductive elimination of the substrate that in turn increases TON (Figure 1). This catalyst is versatile and catalyzes a variety of cross-coupling reactions such as Negishi, Suzuki, Buchwald-Hartwig amination, combined amination/Heck reactions and Kumada couplings with good overall yields.1,2,4
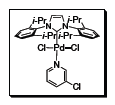
Figure 1.The 3-chloropyridine functions as a ‘throw-away’ ligand, while the bulky IPr ligand improves the reductive elimination of the substrate that in turn increases TON
Scheme 1 illustrates the strong ability of PEPPSI™ catalyst to effect cross-couplings (sp2–sp2 Negishi) under mild reaction conditions. The aryl bromide was completely converted to 4-methyl-4′- methoxylbiphenyl in two hours at room temperature, whereas alternative Pd systems require overnight reaction times to reach adequate conversions. Another compelling feature of the PEPPSI™ system is the low (1 mol %) loadings in Negishi couplings, wherein sp3–sp3 couplings have been achieved in short (30 min) reaction times with high conversions. Previous NHC protocols involving alkyl–alkyl coupling reactions had not been accomplished successfully in high yield.
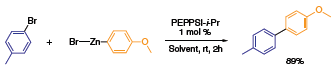
Scheme 1.The strong ability of PEPPSI™ to effect cross-couplings (sp2–sp2 Negishi) under mild reaction conditions.
Negishi Substrate Scope: sp3−sp2 couplings
The range of substrates successfully applied in the Negishi reaction for sp3(RX)–sp2(RZnX) couplings includes both electron donating and electron withdrawing substituents on the arylzinc reaction partner. It is worth noting the coupling of chiral (S)-citronellyl bromide in which the final product does not show an observable erosion of enantiopurity (compound 1). The mild nature of the PEPPSI™ catalyst tolerates pendant alkenyl and alkynyl functional groups as well as the often sensitive TMS group (2–3). Isolated product yields in this coupling class are all greater than 80%.
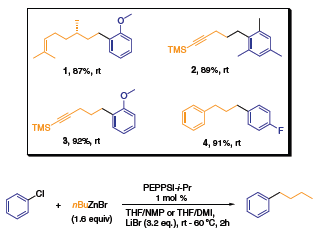
Scheme 2.The range of substrates successfully applied in the Negishi reaction for sp3(RX)–sp2(RZnX) couplings includes both electron donating and electron withdrawing substituents on the arylzinc reaction partner.
Buchwald-Hartwig Amination
The indole moiety is one important element in organic compounds that exhibit pharmacological activity. The most popular method utilized for indole synthesis is the Fischer indole synthesis, wherein an N-acyl hydrazone is transformed into the indole architecture through a sigmatropic rearrangement. As a complement to that well-known methodology, the Organ group has reacted a vinyl halide with various 2-bromoanilines in the presence of PEPPSI™ to afford 2-substituted indoles in good yields (Scheme 3).4 This elegant strategy is being applied toward the expeditious preparation of N-alkyl and N-aryl-indoles for combinatorial libraries.
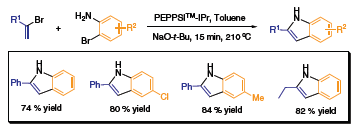
Scheme 3.Buchwald-Hartwig Amination
References
To continue reading please sign in or create an account.
Don't Have An Account?