ATRP: Complete Tools for the Synthesis of Well-Defined, Functionalized Polymers
Wojciech Jakubowski, Nicolay V. Tsarevsky, Patrick McCarthy
ATRP Solutions, Inc., 166 N. Dithridge Street, Suite G4, Pittsburgh, PA 15213
Synthesis of functional polymers via ATRP
Atom transfer radical polymerization (ATRP) provides a simple route to many well-defined (co)polymers with predetermined molecular weight, narrow molecular weight distribution, and high degree of chain end functionality.1-4 It has been effectively applied to the preparation of polymers with precisely controlled functionalities, topologies, and compositions.5, 6 ATRP is a radical process and is much more tolerant of functional groups than ionic polymerizations. This broadens the range of unsaturated molecules that can be polymerized or copolymerized via ATRP and opens the possibilities of straightforward introduction of various functionalities into the polymer structure. Numerous (co)polymers can be formed with a wide-ranging spectrum of properties. As presented in Figure 1, there are four major strategies for the synthesis of telechelic polymers with functional groups via ATRP:7
- Use of functional initiators
- Substitution of the terminal halogen atom
- Direct polymerization of functional monomers
- Polymerization of "protected" monomers, followed by post-polymerization chemical transformations
The first two approaches yield end-functionalized polymers whereas the last two yield polymers with multiple functionalities along the backbone (Figure 1).
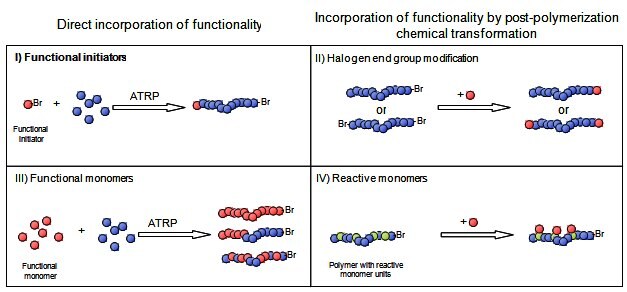
Figure 1.Methods of incorporating functionality into polymers using ATRP.
Functional initiators for ATRP
ATRP uses simple initiators, mainly alkyl halides R-X (X = Cl, Br).1, 8, 9 The number-average molecular weight (Mn) of polymers prepared by ATRP depends on the initial concentration ratio of monomer (M) to initiator as well as the monomer conversion:
Mn = ([M]0 / [RX]0) × conversion × MW(M). The alkyl halides used as initiators can contain one or more halogen atoms. Depending on the exact initiator structure and the number of halogen atoms, the architecture of the prepared polymers can be varied from linear (using alkyl halides with a single halogen atom), to star-like or brush-like (multiple halogen atoms in the initiator). Star polymers can be generated using initiators with alkyl halide groups attached to a single core (Figure 2).
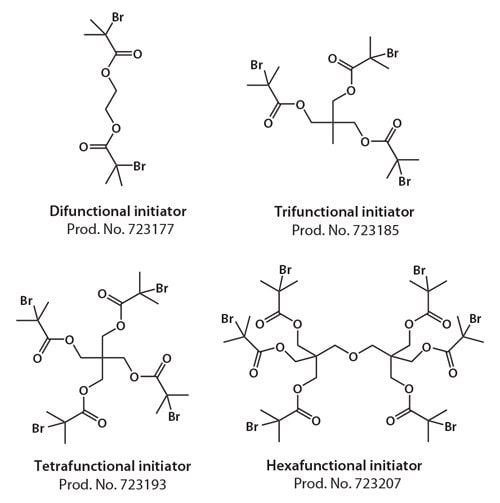
Figure 2.Examples of ATRP initiators yielding polymeric stars.
The alkyl halides used as initiators may also contain various functional groups. The main advantages of using functional initiators in the synthesis of polymers via ATRP are the following:
- Direct functionalization
- No post-polymerization modification required
- Yields α-telechelic polymers
- Multiple applicable functionalities (more than those attainable via nucleophilic substitution of ω-end halogen atom)
Figure 3 illustrates structures of alkyl halide functional initiators that yield end-functionalized polymers. Groups such as hydroxyl in the structure of the ATRP initiator (I) are suitable for the synthesis of polymers that can react with molecules, or surfaces with carboxylic acid / isocyanate groups. Polymers synthesized with initiators containing azide or alkyne groups are able to participate in click chemistry-type functionalizations. Allyl group-containing initiators (III) yield polymers that can undergo hydrosilylation or thiolene reactions with molecules or surfaces containing Si-H or S-H bonds, respectively. Trichlorosilyl groups react with surfaces containing hydroxy or amine groups (including Si-OH bonds, such as those on the surface of silica particles or glass).
Finally, disulfide-containing difunctional initiators (IV) yield polymers containing a functional group able to react with gold surfaces, and gives the ability to degrade in reducing environments. It should be noted that polymers prepared by ATRP contain two chain ends: the α-end derived from the initiator and the ω-end, which is normally bromide or chloride. Alkyl halides (II and V)can participate in a number of nucleophilic substitution reactions, which expands the types of end-functional polymers accessible through ATRP.9
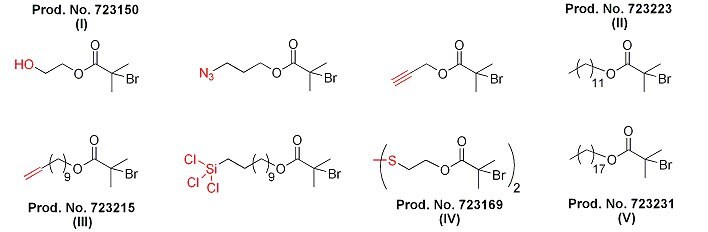
Figure 3.Examples of functionalized alkyl halide initiators for ATRP.
Ligands for highly active ATRP catalysts
One drawback of the classical ATRP method is the use of large amounts of the CuX / ligand catalyst complex required.2, 11 The obtained polymers require tedious purification to remove the catalyst. Although various methods for catalyst removal have been developed, the extra purification step is associated with longer time needed to obtain the final product, and generates chemical waste.12
The use of very active ligands tris[2-(dimethylamino)ethyl]amine (Me6TREN, Prod. No. 723142) and tris(2-pyridylmethyl)amine (TPMA, Prod No. 723134) alleviates this problem (Figure 4a). These ligands can be used in new techniques called Activators ReGenerated by Electron Transfer (ARGET)13, 14 and Initiators for Continuous Activator Regeneration (ICAR),15 which decreases the amount of catalyst needed to only a few (often single-digit) ppm. For comparison, 1,000 to 10,000 ppm of catalyst were used in traditional ATRP. Both techniques employ a reducing agent, a radical initiator such as AIBN15 in ICAR ATRP or tin(II) ethylhexanoate,13, 14, 16-18 ascorbic acid,19 glucose,14 hydrazine,15 or Cu(0),20 in ARGET ATRP. These reducing agents allow for the regeneration of the lower oxidation state metal complex, which would normally be irreversibly converted to the higher oxidation state complex due to radical termination (Figure 4b).
![a) Ligands for Cu-mediated ATRP using ppm amounts of catalyst (Me6TREN - tris[2-(dimethylamino)ethyl]amine; TPMA - tris(2-pyridylmethyl)amine) and b) mechanism of ARGET (and ICAR) ATRP with ppm amounts of catalyst. Ligands for highly active ATRP catalysts](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/materials-science-and-engineering/polymer-synthesis/ppm-amounts-catalyst/ppm-amounts-catalyst.jpg)
Figure 4.a) Ligands for Cu-mediated ATRP using ppm amounts of catalyst (Me6TREN - tris[2-(dimethylamino)ethyl]amine; TPMA - tris(2-pyridylmethyl)amine) and b) mechanism of ARGET (and ICAR) ATRP with ppm amounts of catalyst.
The ARGET and ICAR ATRP processes allow chemists to reduce the amount of catalyst more than one thousand times and the polymers obtained are white or colorless. These processes also allow preparation of well-defined block copolymers,18 polymers with high molecular weight,16, 21 high chain-end functionality16 and adjustable molecular weight distribution.22 In addition, since the ARGET and ICAR ATRP can be performed with the large excess of reducing agent, the reaction can be successfully carried out in the presence of limited amounts of air.17 Reactions can be carried out without deoxygenation, in flasks fitted with rubber septa or even in simple jars. This polymerization process does not require any special equipment and is well-suited for grafting from larger surfaces and can be also applied for preparation of any other polymer materials.
For further background information about the mechanism or the ATRP process as well as several examples of ATRP polymer synthesis, please refer to the ATRP article in the recent Material Matters™ v.5.1.
Synthetic protocol: Example of ICAR ATRP
To verify utility of the commercially available ligands (Prod. No. 723134 or 723142), we prepared polystyrene homopolymer with controlled molecular weight and low polydispersity using ICAR ATRP (Scheme 1). The following is a procedure employing very low concentration of copper catalyst (50 ppm of CuIIBr2/TPMA) and AIBN as reducing agent that yields well-defined polystyrene macroinitiator (PSt-Br) with degree of polymerization ~100.
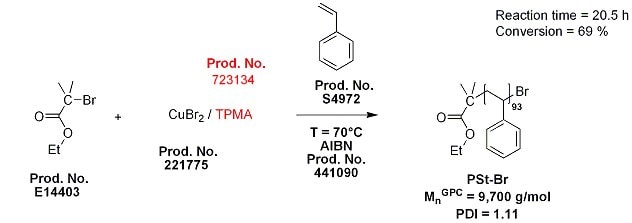
Scheme 1.Synthesis of the polystyrene macroinitiator (PSt-Br) using ICAR ATRP in the presence of the TPMA ligand.
- Add CuBr2 (Prod. No. 221775; 7.8 mg, 3.5 x 10-2 mmol) and TPMA (Prod. No. 723134; 10.1 mg, 3.49 x 10-2 mmol) to a 10-mL flask equipped with magnetic stirring bar.
- Add DMF (Prod. No. 227056; 4ml) to solubilize CuBr2/TPMA. Stir for 10 min to obtain a homogeneous yellowish solution.
- Add St (Prod. No. S4972; 80.0 ml, 69.8 x 10-2 mmol), AIBN (Prod. No. 441090; 0.153 g, 0.0931 mmol) and ethyl 2-bromoisobutyrate (Prod. No. E14403; 0.68 ml, 4.65 mmol) to a 200-mL round bottom flask equipped with a magnetic stirring bar.
- Transfer the catalyst solution to the 200-mL round bottom flask reactor.
- Close the flask reactor with glass adapter (with glass stopcock and a rubber septum). Stir the solution while purging with nitrogen for 1 h.
- Place the flask in an oil bath at 70 °C. To follow the progress of the reaction, samples can be withdrawn with a stainless steel needle. The samples can be analyzed by GC or NMR (monomer conversion) and SEC (molecular weight and polydispersity).
- After 20.5 h*, the monomer conversion reaches 69 %. Mn = 9,700 g/mol, PDI = 1.11. The reactor is opened to air and allowed to cool to room temperature.
- Dilute the polymer with THF (Prod. No. 401757; 100 mL) and precipitate into 2 L of hexane (Prod. No. 296090).
- Dry the produced polymer at 45 °C to constant weight (ca. 24 h).
* Time of the reaction may vary depending on the type of equipment used and purity of chemical reagents.
As shown in Figure 5, the polymerization is well-controlled, the linear first-order kinetic plot of monomer consumption indicates a constant number of active species and the increase of molecular weights with conversion is characteristic of a living process. Molecular weight control is excellent and follows theoretical values based on quantitative initiation. GPC traces are monomodal and shift towards higher molecular weights with polymerization time. The final polymer, after precipitation in hexane, appeared as a white solid powder with only 5 ppm of the residual catalyst.
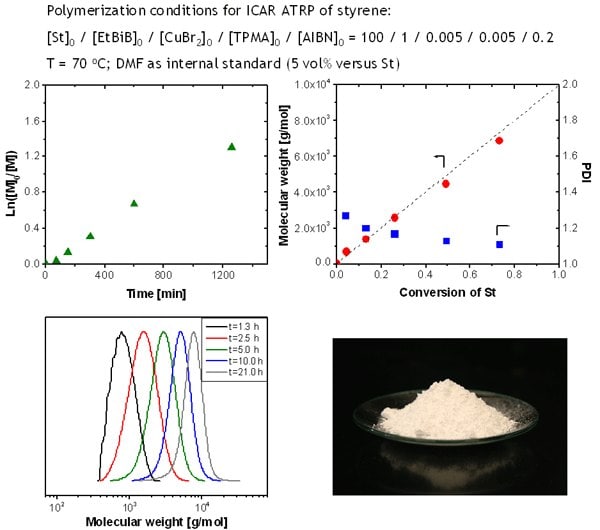
Figure 5.ICAR ATRP of styrene (St) using 50 ppm of catalyst. (a) Kinetic Plot (b) Molecular weight and polydispersity as a function of conversion (c) Evolution of GPC traces (d) Photograph of polymer after precipitation in hexane.
Summary
The properties and application of polymers depend not only on the molecular size, but also on the molecular shape and composition.5 Today, ATRP is one of the most powerful polymer synthetic methods, which allows control over molecular architecture, as evidenced by over a hundred of patent applications and a thousand papers published annually as well as commercial products made in US, Japan and Europe. Due to developments of very active ligands such as Me6TREN and TPMA and recent advancements in initiation processes (ARGET and ICAR ATRP), it is easy to perform any ATRP polymerization reaction and the purification of the final products is now simplified, generating minimum amount of waste.
To continue reading please sign in or create an account.
Don't Have An Account?