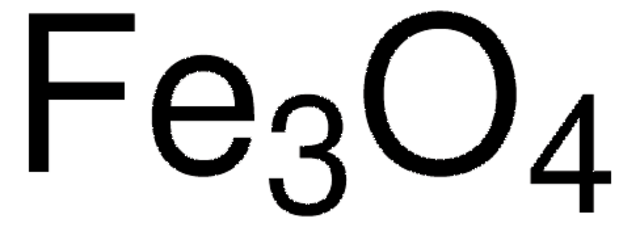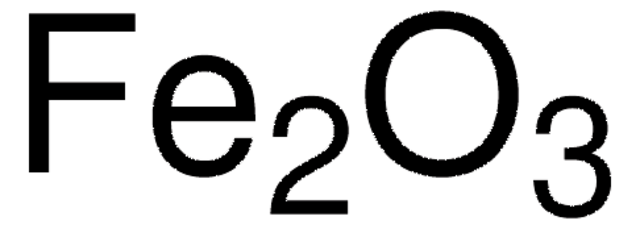推荐产品
检测方案
97% trace metals basis
形式
nanopowder
spherical
表面积
6-8 m2/g , estimated
粒径
50-100 nm (SEM)
mp
1538 °C (lit.)
密度
4.8-5.1 g/mL at 25 °C (lit.)
堆积密度
0.84 g/mL
应用
battery manufacturing
SMILES字符串
O=[Fe].O=[Fe]O[Fe]=O
InChI
1S/3Fe.4O
InChI key
SZVJSHCCFOBDDC-UHFFFAOYSA-N
正在寻找类似产品? Visit 产品对比指南
一般描述
应用
分析说明
储存分类代码
11 - Combustible Solids
WGK
nwg
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
分析证书(COA)
输入产品批号来搜索 分析证书(COA) 。批号可以在产品标签上"批“ (Lot或Batch)字后找到。
已有该产品?
为方便起见,与您过往购买产品相关的文件已保存在文档库中。
难以找到您所需的产品或批次号码?
如何查找产品货号
在网站页面上,产品编号会附带包装尺寸/数量一起显示(例如:T1503-25G)。请确保 在“产品编号”字段中仅输入产品编号 (示例: T1503).
示例
其它示例:
705578-5MG-PW
PL860-CGA/SHF-1EA
MMYOMAG-74K-13
1000309185
输入内容 1.000309185)
遇到问题?欢迎随时联系我们技术服务 寻求帮助
如何查找COA批号
批号可以在产品标签上"批“ (Lot或Batch)字后面找到。
Aldrich 产品
如果您查询到的批号为 TO09019TO 等,请输入去除前两位字母的批号:09019TO。
如果您查询到的批号含有填充代码(例如05427ES-021),请输入去除填充代码-021的批号:05427ES。
如果您查询到的批号含有填充代码(例如 STBB0728K9),请输入去除填充代码K9的批号:STBB0728。
未找到您寻找的产品?
部分情况下,可能未在线提供COA。如果搜索不到COA,可在线索取。
Which document(s) contains shelf-life or expiration date information for a given product?
If available for a given product, the recommended re-test date or the expiration date can be found on the Certificate of Analysis.
How do I get lot-specific information or a Certificate of Analysis?
The lot specific COA document can be found by entering the lot number above under the "Documents" section.
Is Product 637106, Iron(II,III) oxide, stabilized (coated)?
No, product 637106 has not been coated on the surface per the supplier.
What is the particle sizeof Product 637106, Iron(II,III) oxide?
Average particle sizes are 50-100 nm.
How can the nanopowder Iron(II,III) oxide, Product 637106, be dispersed in an aqueous solution?
1. It is recommended to use a neutral buffered solution (approximately pH 7). The pH of the solution is an important factor in achieving a monodispersion of the nanopowder.2. Probe-type ultrasonication is strongly recommended by the manufacturer.3. If the material is not well dispersed by ultrasonication in a neutral buffer, add a surfactant. This will wet the nanoparticle surface. Since the particles are small (less than 50 nm), they have a high surface area to volume ratio. They can therefore absorb gases such as oxygen or carbon dioxide from the air, which would decrease the dispersity of the particles. Degassing the buffer before use may help to eliminate the formation of gas bubbles.
How do I find price and availability?
There are several ways to find pricing and availability for our products. Once you log onto our website, you will find the price and availability displayed on the product detail page. You can contact any of our Customer Sales and Service offices to receive a quote. USA customers: 1-800-325-3010 or view local office numbers.
What is the Department of Transportation shipping information for this product?
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
My question is not addressed here, how can I contact Technical Service for assistance?
Ask a Scientist here.
商品
Professor Randal Lee (University of Houston, USA) discusses design considerations for iron oxide magnetic nanospheres and nanocubes used for biosensing, including synthetic procedures, size, and shape. The effects of these variables are discussed for various volumetric-based and surface-based detection schemes.
Magnetic nanoparticles have attracted tremendous attention due to their novel properties and their potential applications in magnetic recording, magnetic energy storage and biomedicine.
Magnetic nanoparticles have attracted tremendous attention due to their novel properties and their potential applications in magnetic recording, magnetic energy storage and biomedicine.
Currently, magnetic nanoparticles (MNPs) are attracting a lot of attention because of the possibility of many novel applications, especially in biomedical research.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门