Carbohydrate-Catalyzed Enantioselective Alkene Diboration
Transforming Unsaturated Hydrocarbons into Chiral Building Blocks
Enantioselective diboration of olefins is a valuable strategy for transforming unsaturated hydrocarbons into useful chiral building blocks. Typically, the process is carried out via transition-metal based activation of diboron reagent. Prof. James Morken has developed metal-free, affordable, enantioselective, 1,2-diboration of alkenes cocatalyzed by carbohydrate-derived catalysts and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, 139009). This process employs pseudoenantiomeric glycols, 6-tert-butyldimethylsilyl-1,2-dihydroglucal (THS-DHG, 901235) and dihydrorhamnal (DHR, 901237), derived from D-glucal and L-rhamnal, respectively as chiral catalysts to enhance the reactivity of the diboron nucleus and to control the facial selectivity of the diboration reaction. Among various organoboronic esters studied for this reaction, 2,2′-bi-1,3,2-dioxaborinane (B2(pro)2, 901464) was found to be versatile in promoting reaction efficiency while maintaining high enantioselectivity. Reaction byproducts arising from B2(pro)2, namely, 1,3-propanediol and boric acid are relatively innocuous and easily removable by an aqueous wash. Carbohydrate-derived catalysts, TBS-DHG and DHR, offer not only greener means but also promote highly enantioselective 1,2-diboration of both terminal and internal alkenes at a modest temperature and a shorter reaction time.
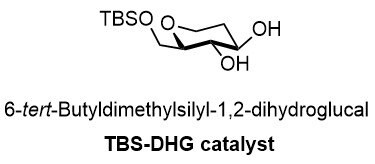
THS-DHG catalyst 901235

DHR catalyst 901237
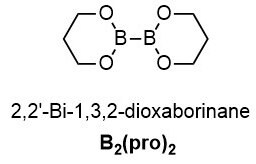
B2(pro)2 901464
Advantages of Carbohydrate-Derived Catalysts
- Operationally simple, greener, and scalable protocol
- Highly enantioselective products from a broad range of alkenes
- Innocuous water-soluble byproducts facilitate clean reactions
- Requires low catalyst loading, moderate temperature, and a short reaction time
Procedure for the Enantioselective Diboration of Alkenes
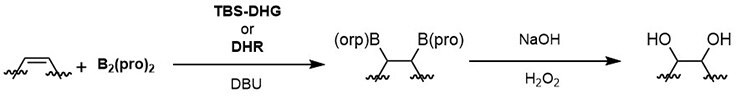
Scheme 1:General scheme for the enantioselective diboration of alkenes with B2(pro)2 and carbohydrate-derived catalysts, TBS-DHG and DHR.
- Charge TBS-DHG or DHR catalyst (0.10–0.15 eq), propanediol diboron, B2(pro)2 (2.0–3.0 eq), 4A molecular sieves, and the alkene substrate (1.0 eq) into a dry reaction flask in the glove box.
- Dissolve the above in ethyl acetate or THF, and add 0.10–0.15 eq of DBU.
- Stir the reaction mixture for 12h (at room temperature or 40 0C).
- Cool the reaction mixture to 0 0C, dilute with the solvent, and add 3 M sodium hydroxide solution followed by dropwise addition of 30% hydrogen peroxide.
- Warm to room temperature, stir for another 4h.
- Cool to 0 0C and add saturated aqueous sodium thiosulfate, dropwise over 5 min.
- Dilute with ethyl acetate and separate the organic and aqueous layers. Extract the organic layer, dry over sodium sulfate, and evaporate the solvents to afford the final product.
Scheme of Enantioselective Diboration of Internal and Terminal Alkenes
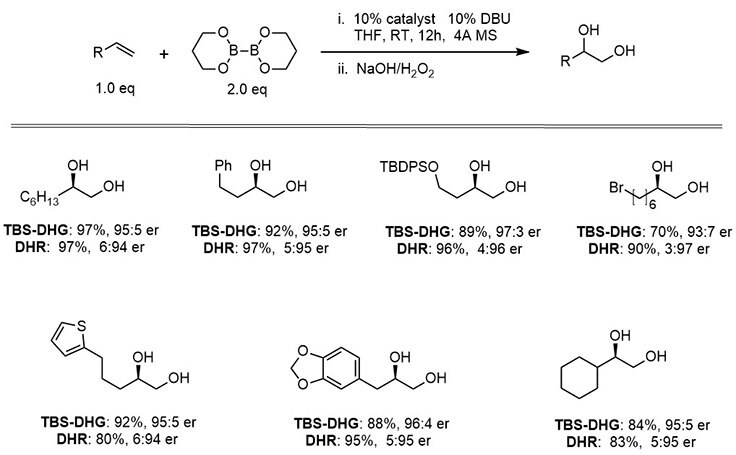
Scheme 2:Enantioselective diboration of terminal alkenes by carbohydrate-derived catalysts
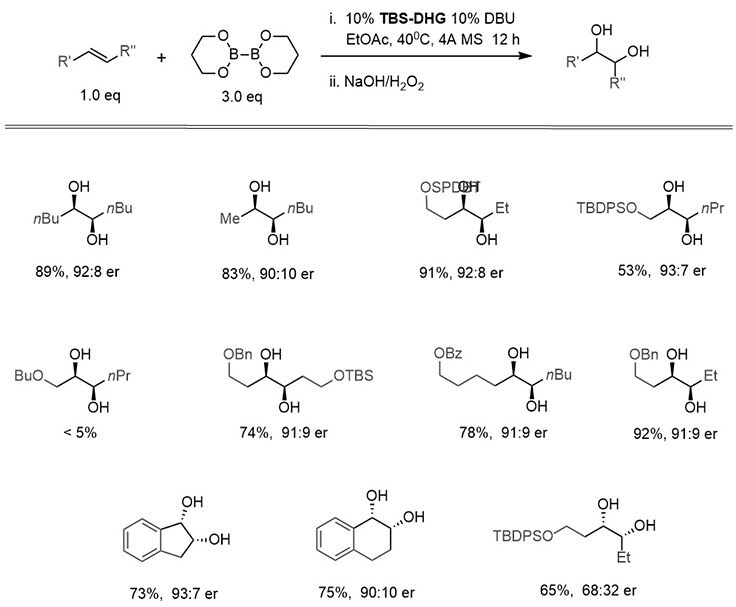
Scheme 3:Enantioselective diboration of internal alkenes by a carbohydrate-derived catalyst, TBS-DHG
References
如要继续阅读,请登录或创建帐户。
暂无帐户?