MIDA Boronates
Introduction
The Suzuki-Miyaura cross-coupling reaction of boronic acids is one of the most important and highly utilized reactions in the organic chemistry toolbox, with applications in polymer science as well as in the fine chemicals and pharmaceutical industries. However, many boronic acids are extremely unstable and susceptible to decomposition that renders them inefficient in coupling reactions or makes long-term storage difficult. Additionally, performing iterative Suzuki Miyaura cross-coupling under mild conditions has not been developed. Recently, Professor Martin Burke at the University of Illinois, Urbana-Champaign developed a method to attenuate the transmetalation between boronic acids and the palladium species by rehybridizing the boron center from sp2 to sp3 via complexation with a trivalent N-methyliminodiacetic acid (MIDA) ligand. This allowed for the possibility of performing sequential Suzuki-Miyaura reactions under mild conditions.
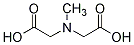
MIDA M51008
The MIDA-protected boronate esters are easily handled, indefinitely bench-top stable under air, compatible with chromatography, and unreactive under standard anhydrous cross-coupling conditions, even at temperatures up to 80 °C. However, deprotection is easily achieved at room temperature under mild aqueous basic conditions using either 1M NaOH, or even NaHCO3.
Reference: Gillis, E. P.; Burke, M. D. J. Am. Chem. Soc. 2007, 129, 6716.
View a complete listing of MIDA Boronates from Sigma-Aldrich.
Representative Applications
MIDA as a Protecting Group and Iterative Cross-Coupling
To demonstrate the efficacy of MIDA as a protecting group, Burke’s group reacted a 1:1 mixture of the MIDA boronate (698229) and 4-butylphenylboronic acid with 4-bromobenzaldehyde under Buchwald’s anhydrous Suzuki-Miyaura conditions (Scheme 1). The resultant product mixture displayed a 24:1 preference for reaction with the unprotected boronic acid. Conversely, when a control reaction was performed with a mixture of 4-n-butylphenylboronic acid and either the unprotected boronic acid (393622) or the N-methyldiethanolamine adduct, no selectivity was observed.
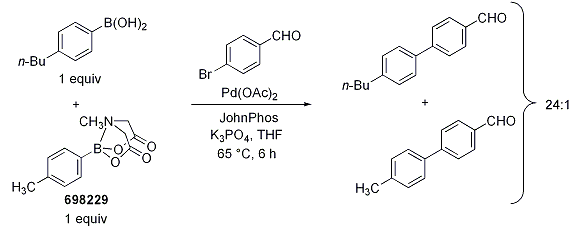
Scheme 1
The potential for iterative cross-couplings using the Burke methodology was demonstrated in their total synthesis of ratanhine (Scheme 2). trans-1-Propen-1-ylboronic acid was coupled with the benzofuranyl MIDA boronate 1, which was deprotected and cross-coupled with the bulky aryl bromide MIDA boronate 2 at elevated temperature. The subsequent intermediate was deprotected, coupled with vinyl bromide 3, to yield the diMOM ether, and cleavage of the two MOM groups resulted in ratanhine. Enabling features of this type of synthesis include the use of only a single, mild reaction to assemble a collection of easily synthesized, readily purified, and highly robust building blocks. Moreover, the short and modular nature of this pathway enables the easy preparation of analogs simply by substituting modified building blocks into the same iterative cross-coupling sequence.
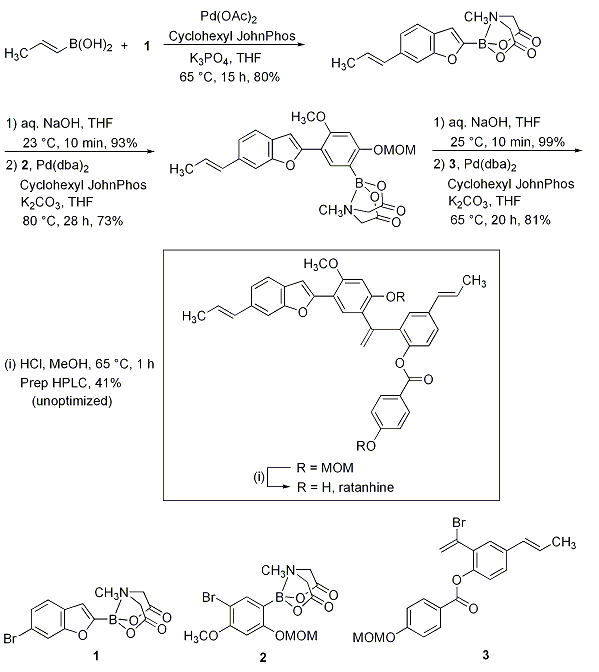
Scheme 2.
MIDA Boronates of Polyenes
Typically, polyenylboronic acids are very unstable and therefore not employed in synthesis. In another exemplary demonstration of the MIDA boronates’ stability and efficiency in iterative cross-coupling, Burke and coworkers utilized a common alkenyl MIDA boronate (703478) to create a series of polyenyl building blocks. The MIDA boronate terminus is inert to Heck, Stille, and Suzuki couplings, yielding butadienyl MIDA boronates (Scheme 3).
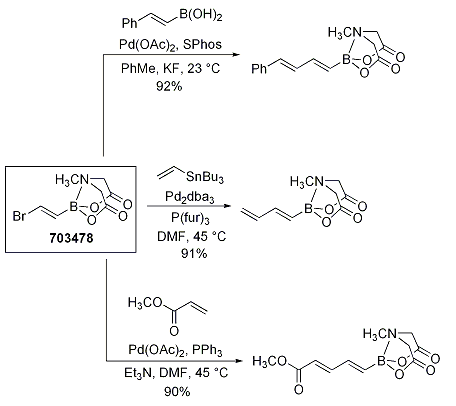
Scheme 3
The alkenyl MIDA boronate 703478 was also applied to the synthesis of the carotenoid all-trans-retinal. Demonstrating the feasibility of polyenyl MIDA boronates in synthesis, the B-deprotection of the intermediate 4 proceeded smoothly to generate the boronic acid, which was subsequently coupled with the β-bromo enal to provide all-trans-retinal (Scheme 4).
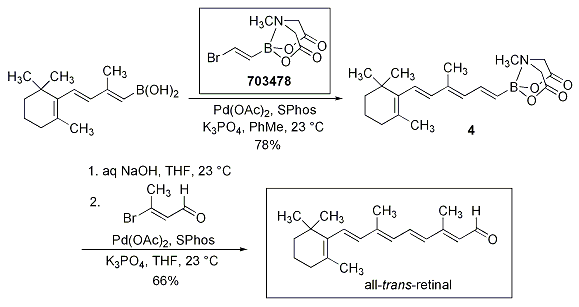
Scheme 4
Even more impressive are Miyaura borylation, Sonogashira and Negishi couplings that yield bis-metalated lynchpin-type reagents (Scheme 5). The reagents 5 and 6 were further elaborated in polyene natural product syntheses of β-parinaric acid (Scheme 6) and the polyene chain of amphotericin B via the stable polyene building block 7 (Scheme 7).
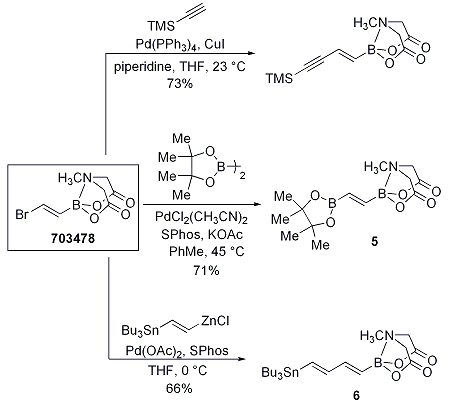
Scheme 5
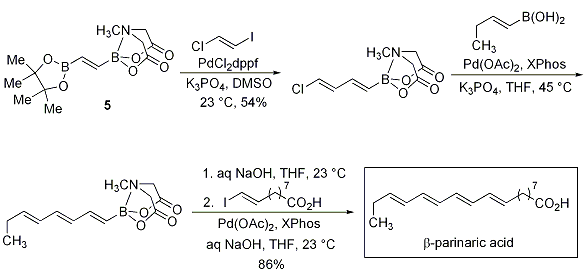
Scheme 6
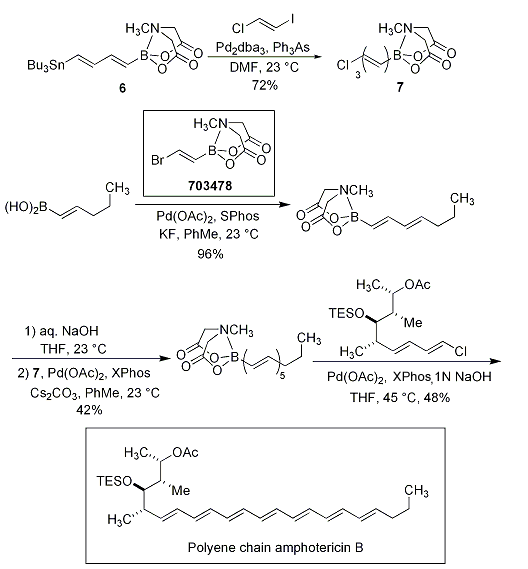
Scheme 7
Formation of Complex Boronic Acids from MIDA Boronates
Another extremely useful feature of the MIDA boronates is their compatibility with a wide range of common synthetic reagents, allowing for the elaboration of functionalized MIDA boronates to create structurally complex boronic acid surrogates. This was recently demonstrated by Burke and coworkers with the transformation of the 4-(hydroxymethyl)phenyl MIDA boronate (698105) by a variety of common oxidants and reagents to create an array of synthetically useful MIDA boronates (Scheme 8). Even harsh reagents such as triflic acid, and Jones oxidation were well tolerated, keeping the MIDA boronate intact. In several cases, transformations can be easily reversed to yield the original 4-(hydroxymethyl)phenyl MIDA boronate.

Scheme 8
The 4-formylphenyl MIDA boronate 697494 was further elaborated in various C-C bond-forming reactions. The results show that MIDA boronates are also compatible with reductive amination, Evans aldol, Horner-Wadsworth-Emmons olefination, and Takai olefination protocols (Scheme 9).
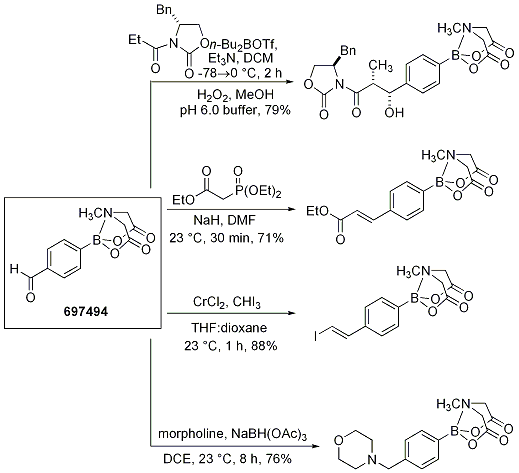
Scheme 9
The impact of the exceptional compatibility of the MIDA boronate was exhibited in the total synthesis of (+)-crocacin C starting from an acrolein MIDA boronate (Scheme 10). The MIDA boronate tolerated a Paterson aldol and diastereoselective reduction protocol to yield the diol MIDA boronate, which was purified by silica gel chromatography. The purified MIDA boronate was permethylated with Meerwein’s salt, deprotected with CAN, oxidized with Dess-Martin periodinane and subjected to Takai olefination to yield the stable, crystalline, complex MIDA boronate 8. Stille coupling of 8 with known building block 9, followed by in situ boronic acid release and cross-coupling with bromobenzene provides (+)-crocacin C.
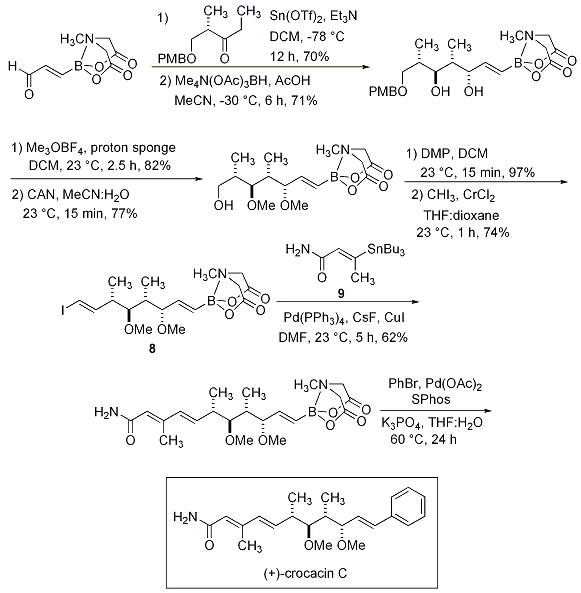
Scheme 10
Preparation of trans-(2-bromovinyl) MIDA Boronate and Vinyl MIDA Boronate from the Corresponding Silanes
Due to the instability of some boronic acids, the Burke group has developed very practical syntheses of more sensitive building blocks. For instance, while trans-(2-bromovinyl) MIDA boronate (703478) could be prepared via bromoboration of acetylene and reaction with MIDA in the presence of base, a more convenient procedure was subsequently developed. The synthesis of trans-(2-bromovinyl) MIDA boronate is achieved via transmetalation of 1-bromo-2-trimethylsilylethylene with BBr3, followed by trapping with MIDA2-Na+2 to form the MIDA boronate (Scheme 11-(1)). Likewise, formation of vinyl MIDA boronate was achieved according to this procedure to give rise to the very useful vinyl MIDA boronate building block (Scheme 11-(2)). Of note, the corresponding boronic acid is very unstable.
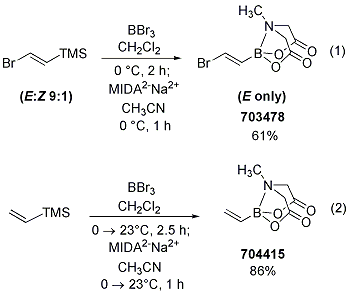
Scheme 11
Synthetic Utility of Vinyl MIDA Boronate
The utility of vinyl MIDA boronate was demonstrated via cycloproponation and epoxidation to the corresponding MIDA cyclopropane and oxirane, respectively. These procedures (Scheme 12) provided air and chromatography-stable solids in both cases, with the oxirane being the first known synthesis of an unsubstituted oxiranylborane (confirmed by X-ray analysis of the oxiranylborane).
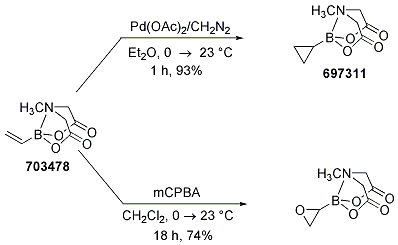
Scheme 12
Vinyl MIDA boronate was also successfully subjected to Heck and oxidative Heck reactions as well as to olefin metathesis (Scheme 13) to provide the cross-coupled product. The cross metathesis of vinyl MIDA boronate with various olefins represents a potent strategy for the highly stereoselective construction of substituted vinyl boranes. This method proved successful in the preparation of a variety of disubstituted olefins (Table 1) with excellent yields (81-98%) and diastereoselectivities (>20:1) and represents a significant advance since this method was often limited by the stability of the alkenyl boronic esters, the diastereoselectivity of the transformation, or the ease in purification of the product disubstituted alkenyl borane.
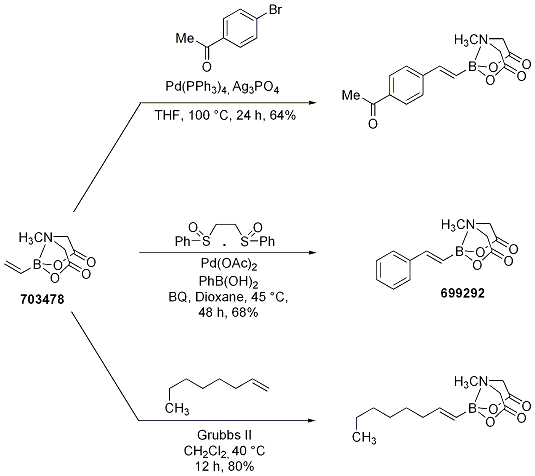
Scheme 13
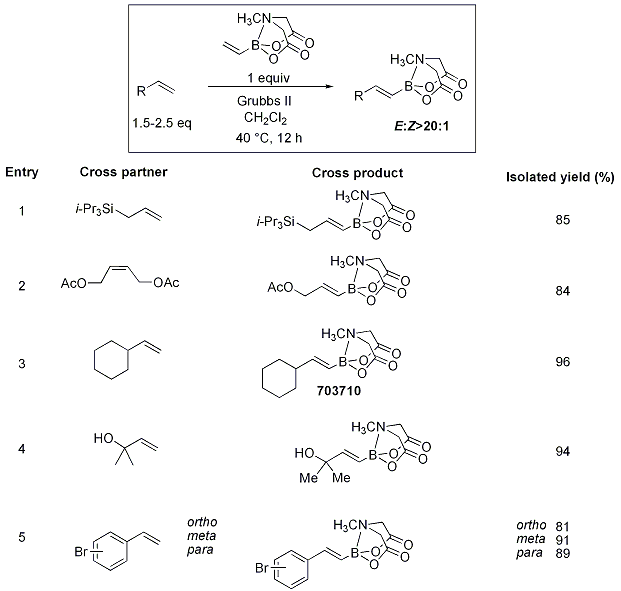
Table 1.Cross-Metathesis with Vinylboronic acid MIDA ester
Slow-Release of Unstable Boronic Acids from MIDA Boronates
In addition to the attenuated reactivity towards anhydrous cross-coupling, MIDA boronates possess the highly-enabling capacity for in situ slow-release of boronic acids under aqueous basic conditions. Harnessing this phenomenon, boronic acids that are notoriously unstable can be effectively utilized in cross coupling when employed as MIDA boronates. The slow-release of more sensitive boronic acids can be achieved using K3PO4. While aqueous solutions of NaOH promote the fast hydrolysis to the corresponding boronic acid, the use of aqueous K3PO4 allows for the slow-release of the relatively unstable boronic acid, which prevents decomposition of the precursor organometallic and improves overall yield of the Suzuki reaction.
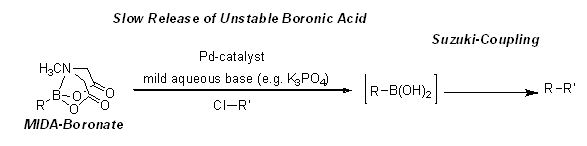
Figure 1. Slow-Release of Unstable Boronic Acids
- Air-stable
- Stable to anhydrous cross-coupling conditions
- Compatability with common and harsh synthetic reagents
- Solubility in various organic solvents
- Monomeric composition
- Silica-gel compatible
- Ability to undergo slow release cross-coupling
Burke and coworkers examined the slow-release concept by comparing various freshly prepared boronic acids with the corresponding MIDA boronates, considering both the benchtop stability and efficiency in the cross-coupling. The study revealed that many boronic acids decompose significantly via various methods, including protodeboronation, oxidation, and polymerization (after just 15 days benchtop storage under air-Table 2). On the other hand, the corresponding MIDA boronates were remarkably stable, with >95% of each MIDA remaining after ≥60 days benchtop storage under air. In addition to complications related to the storage of various classes of boronic acids, efficiency of the Suzuki reaction is also impacted by the nature of the boron-unit since boronic acids decompose in situ faster than the cross-coupling can occur. Further complicating this scenario is that decomposition is accelerated in the presence of reagents and conditions common to many Suzuki reactions. Consistent with this analysis, the yields observed when even freshly-prepared boronic acids are employed in cross-coupling reactions are generally low to moderate. In stark contrast, when the MIDA boronates were subjected to the same reaction conditions, conversion to the cross-coupled product was achieved with excellent efficiency (Table 2).
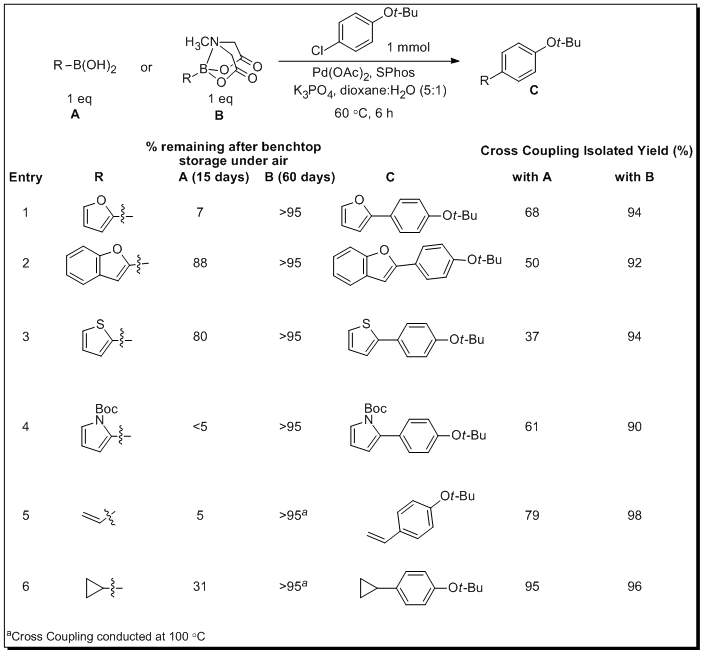
Table 2.Comparison of Benchtop Stability and Coupling Efficiency of Boronic Acids and MIDA Boronates
The scope of this new phenomenon was further investigated utilizing various MIDA boronates of which the corresponding boronic acids have historically exhibited challenges with respect to either storage or usage. Table 3 shows a range of MIDA boronates that are highly effective in Suzuki-Miyaura cross-coupling reactions with unactivated (and in many cases either electronically or sterically deactivated) aryl and heteroaryl chlorides. In addition to 2-substituted heteroaryl MIDA boronates, vinyl MIDA boronate as well as cyclopropyl MIDA boronate were utilized under slow-release conditions to effect difficult couplings with deactivated heteroaryl chlorides. The unprotected boronic acids are generally known to be poor substrates for cross-coupling, but when generated from the corresponding MIDA boronates under slow-release conditions, they become viable synthetic candidates, providing the cross-coupled product in excellent yields.
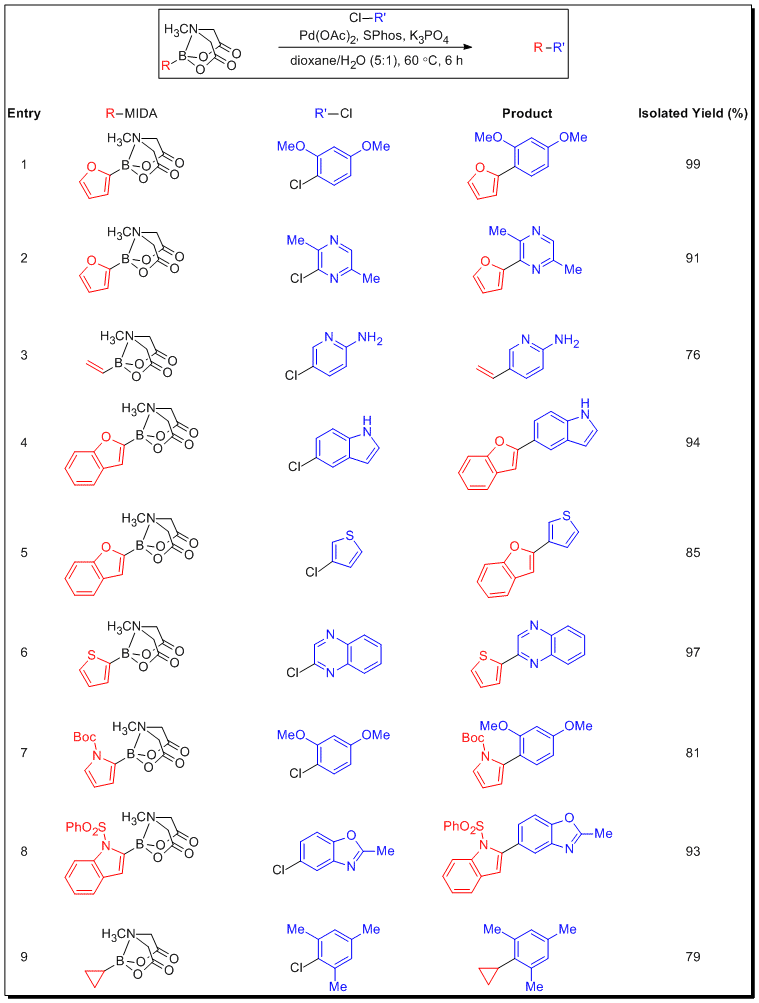
Table 3.Slow Release Cross-Coupling of MIDA boronates
The development of an air-stable and effective surrogate for the notoriously unstable 2-pyridyl boronic acid has been a long-standing challenge in the field of cross-coupling. This motif is ubiquitous in drug-like small molecules, which makes this problem particularly important. While 2-pyridyl boronic acid surrogates exist, their use is complicated by their air- and moisture-sensitivity as well as their somewhat variable and impure composition. In contrast, Burke and coworkers found that the 2-pyridyl MIDA boronate is isolable, benchtop stable under air, and chromatography stable. While conditions that promoted the slow release cross-coupling noted above were not generally effective for 2-pyridyl MIDA boronate, the utilization of isopropanol instead of water as co-solvent and a sub-stoichiometric amount of Cu(OAc)2 as co-catalyst (copper salts have been shown previously to promote challenging Suzuki-Miyaura couplings) proved to be successful. In addition to the unprecedented benchtop stability of the 2-pyridyl MIDA boronate (X-ray structure obtained), the slow-release of the active boron species allows for successful coupling with a variety of aryl and heteroaryl chlorides (Table 4).
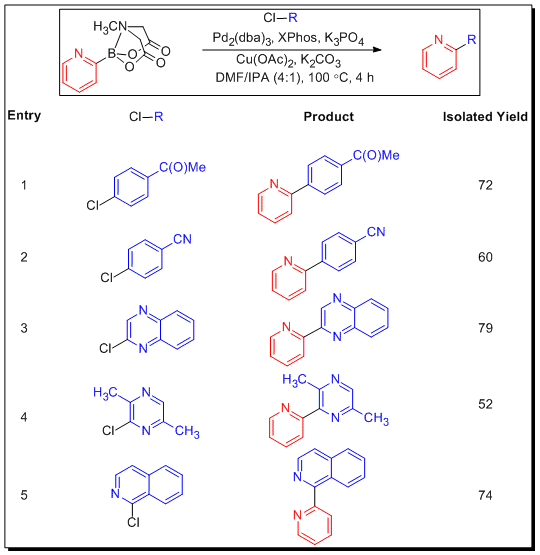
Table 4.Slow Release Cross-Coupling of 2-pyridyl MIDA boronate
Discover MIDA Boronates (1 Mb PDF)
View our complete listing of MIDA Boronates.
For more information, please see the following publications:
如要继续阅读,请登录或创建帐户。
暂无帐户?
