Ir(I)-Catalyzed C–H Borylation for Rapid Synthesis of Arylboronic Esters
Arylboronic acids and esters are used for a variety of transformations, most notably the Suzuki-Miyaura cross-coupling reaction. This reaction is used to selectively construct C–C bonds through the combination of an organo-boron nucleophile with a suitable aryl, alkenyl, or alkyl halide or triflate. While the Suzuki-Miyaura reaction has become commonplace within the synthetic community, one limitation of this method is the limited ability to access the requisite organo-boron species.
Historically, methods for the synthesis for aryl C–B bonds have relied upon the use of harshly basic reaction conditions or substrates containing prefunctionalized carbon centers. These shortcomings require additional steps to either protect sensitive functionality or install the necessary functional handle before C–B bond formation (Figure 1).

Figure 1.Classical methods for C–B bond formation
The direct formation of aryl C–B bonds from aryl C–H bonds thus represents a powerful strategy for streamlining the synthesis of these useful reagents (Figure 2).1

Figure 2.Metal-catalyzed direct C–H borylation
Building upon his previous work within the area,2 Professor John F. Hartwig developed a method for directly converting aryl C–H bonds to aryl C–B bonds using an Ir(I) catalyst and B2pin2 (Figure 3).3 This powerful system displays excellent regioselectivity that can be predicted by sterics and leads to the rapid synthesis of highly useful arylboronic esters.
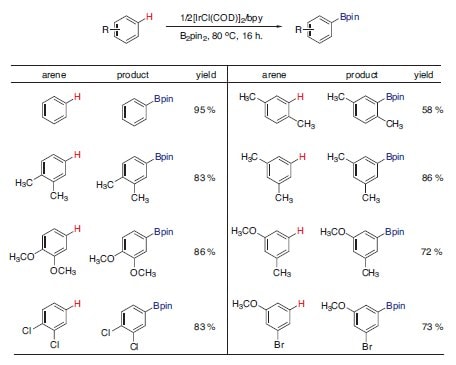
Figure 3. Ir(I)-Catalyzed aryl C–H borylation
This method provides a simple and direct route to arylboronic esters that fully avoids the use of harshly basic reaction conditions and does not require multiple reaction steps and manipulations. Importantly, this reaction uses catalysts and reagents that are readily available.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?