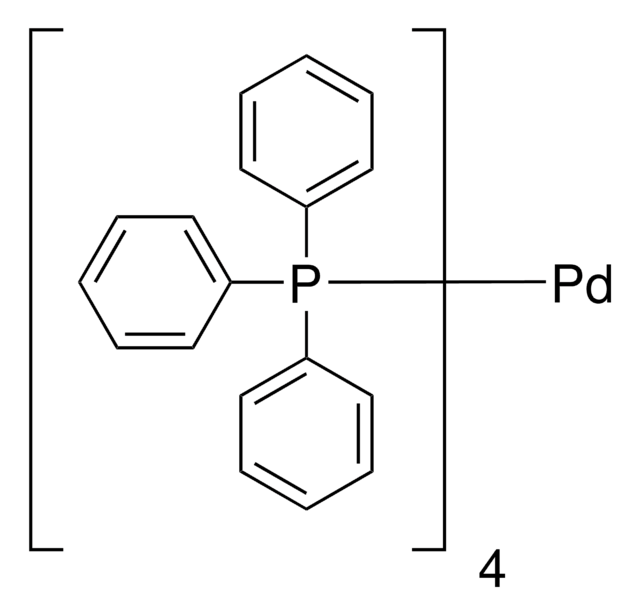
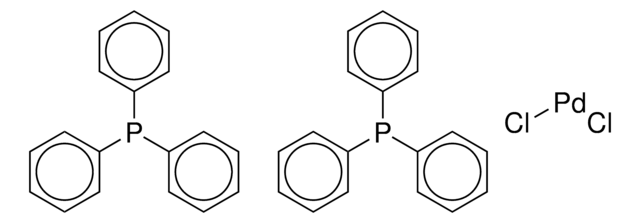
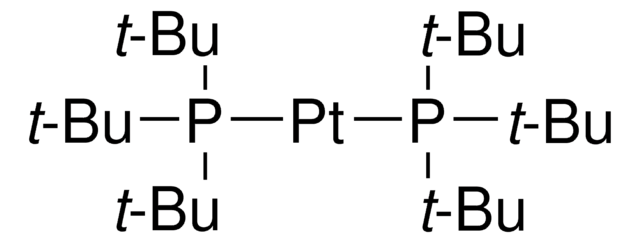Recommended Products
form
solid
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
mp
258-272 °C
greener alternative category
storage temp.
−20°C
SMILES string
[Pd].CC(C)(C)P(C(C)(C)C)C(C)(C)C.CC(C)(C)P(C(C)(C)C)C(C)(C)C
InChI
1S/2C12H27P.Pd/c2*1-10(2,3)13(11(4,5)6)12(7,8)9;/h2*1-9H3;
InChI key
MXQOYLRVSVOCQT-UHFFFAOYSA-N
General description
Application
- Catalyst for Suzuki coupling on a multisubstituted sp3-carbon (eq. 1)
- Catalyst for Stille coupling reaction of aryl chlorides (eq. 2)
- Catalyst for Negishi coupling reaction (eq. 3)
- Catalyst for Heck coupling to form tetrasubstituted olefins (eq. 4)
- Catalyst for Buchwald-Hartwig amination of aryl halide (eq. 5)
- Catalyst for carbonylation of aryl halides with carbamoylsilanes (eq. 6)
On the Way Towards Greener Transition-Metal-Catalyzed Processes as Quantified by E Factors
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Documents related to the products that you have purchased in the past have been gathered in the Document Library for your convenience.
Difficulty Finding Your Product Or Lot/Batch Number?
How to Find the Product Number
Product numbers are combined with Pack Sizes/Quantity when displayed on the website (example: T1503-25G). Please make sure you enter ONLY the product number in the Product Number field (example: T1503).
Example:
Additional examples:
705578-5MG-PW
PL860-CGA/SHF-1EA
MMYOMAG-74K-13
1000309185
enter as 1.000309185)
Having trouble? Feel free to contact Technical Service for assistance.
How to Find a Lot/Batch Number for COA
Lot and Batch Numbers can be found on a product's label following the words 'Lot' or 'Batch'.
Aldrich Products
For a lot number such as TO09019TO, enter it as 09019TO (without the first two letters 'TO').
For a lot number with a filling-code such as 05427ES-021, enter it as 05427ES (without the filling-code '-021').
For a lot number with a filling-code such as STBB0728K9, enter it as STBB0728 without the filling-code 'K9'.
Not Finding What You Are Looking For?
In some cases, a COA may not be available online. If your search was unable to find the COA you can request one.
Articles
TPGS-750-M, a second generation surfactant, is useful for room temperature, palladium and ruthenium-catalyzed reactions in water. Reactions include the Heck reaction, Suzuki-Miyaura reaction, Sonogashira reaction, Buchwald-Hartwig amination reaction, Negishi reaction, and olefin metathesis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
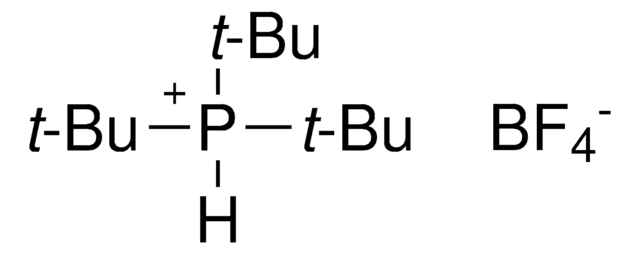
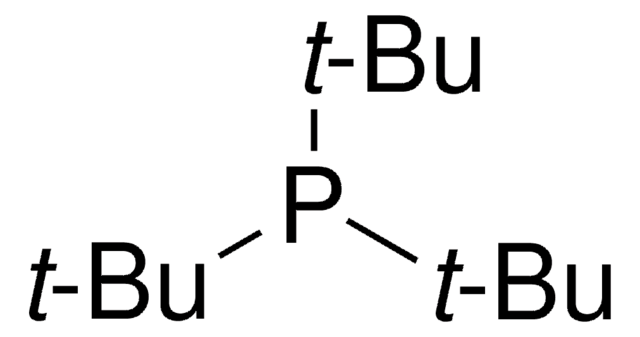
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)