Solvias® Ligands for Enantioselective Hydrogenation
Solvias Ligand products have roots in the development of the Josiphos ligands and the successful implementation in the (S)-metolachlor process—the largest industrial enantioselective manufacturing process—in the former Ciba-Geigy. Several ligand families were added, either via Solvias' own research or via licensing from other companies.1 The central concept for all Solvias ligands is their modularity, usually via the introduction of different phosphino moieties in the last stages of their synthesis. This allows the easy tuning of the steric and electronic properties and, thereby, the adaptation to the needs of a specific reaction. All ligands in the Solvias portfolio are available on a research scale with short lead times; several ligands are being produced regularly in multi-kilogram quantities. The number of ligands within the different families varies from 10 to >100; therefore, it is not practical to have all of them available. We offer a selection representing different steric and electronic properties. Further derivatives for optimization or fine tuning are usually available on request from Solvias.
MeOBIPHEP
In many respects, the catalytic profile of the MeOBIPHEP ligands is similar to other atropisomeric diphosphines such as BINAP and its many analogs.1–3 The nature of the PR2 group strongly influences the catalytic performance of the metal complexes, and the ligands available have different steric bulk.1 MeOBIPHEP complexes are highly effective for the hydrogenation of α- and β-functionalized ketones (Figure 1), the hydrogenation of allylic alcohols and α,β-unsaturated esters (Figure 2), the hydrogenation of heteroarenes (Figure 3) and a variety of synthetically useful C–C coupling reactions (Figure 4).
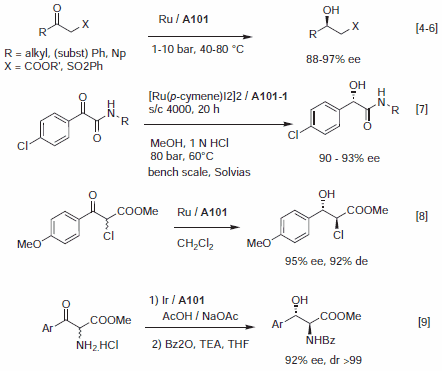
Figure 1.
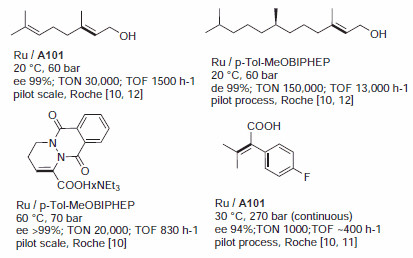
Figure 2.
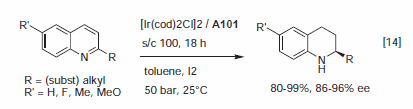
Figure 3.
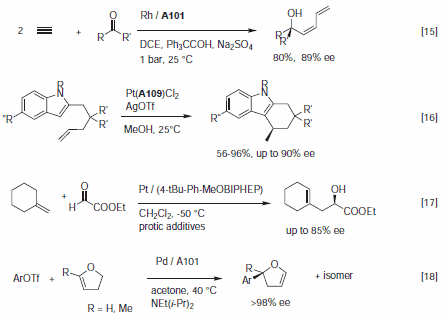
Figure 4.
Josiphos
The Josiphos ligands constitute one of the most versatile and successful ligand families, second probably only to the BINAP ligands. Since the two phosphine groups are introduced in consecutive steps with high yields,1,2 a variety of ligands are readily available with widely differing steric and electronic properties. Up to now, only the (R,SFc)-family (and its enantiomers) but not the (R,RFc) diastereomers have led to high enantioselectivities. At present, about 150 different Josiphos ligands have been prepared and 40 derivatives are available in a ligand kit for screening and on a multikilogram scale for production.3
Catalytic applications of the Josiphos ligand family were reviewed up to 2002.3 Josiphos ligands have been applied in four production processes and 5-6 pilot and bench scale processes involving Rh, Ir, and Ru catalyzed hydrogenation reactions of C=C and C=N bonds.4 Selected applications are summarized in Figure 1. The most important application is undoubtedly the Ir/J005‑1 catalyzed hydrogenation of a hindered N-aryl imine of methoxyacetone, the largest known enantioselective process operated for the enantioselective production of the herbicide (S)-metolachlor.2,5 The hydrogenation of tetrasubstituted olefins was the key step for two production processes developed for the synthesis of biotin by Lonza and of methyl dihydrojasmonate by Firmenich.6 Pilot processes were developed by Lonza for a building block of crixivan and for dextromethorphane.6 Chemists have reported the unprecedented hydrogenation of unprotected dehydro β-amino acid derivatives catalyzed by Rh-Josiphos with ee's up to 97%. It was shown that not only simple derivatives but also the complex intermediate for MK-0431 depicted in Figure 1 can be hydrogenated successfully. Regular production on a multiton/year scale with ee's up to 98% has been started in 2006.7
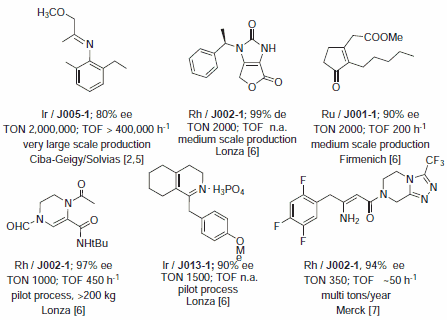
Figure 1.
Rh and Ir complexes with chiral Josiphos ligands are highly selective, active, and productive catalysts for various enantioselective reductions. High enantioselectivities were described for the enantioselective hydrogenation of enamides, itaconic acid derivatives, acetoacetates as well as N-aryl imines (in presence of acid and iodide) and phosphinylimines.3 Josiphos J001 is the ligand of choice for the Cu catalyzed reduction of activated C=C bonds with polymethylhydrosiloxane (PMHS) (Figure 2) with high enantioselectivities for nitro alkenes,8 α,β-unsaturated ketones,9a esters,9b,c and nitriles.10 Recently, it was disclosed that Ru-Josiphospyridinyl- alkylamines complexes are effective for the enantioselective transfer hydrogenation of aryl ketones with turnover frequencies for full conversion exceeding 20,000 h-1 (Figure 3).11
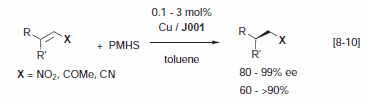
Figure 2.
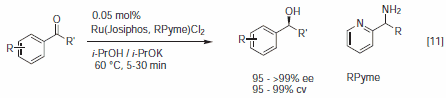
Figure 3.
Josiphos complexes have been successfully applied to various asymmetric catalytic coupling reactions such as allylic alkylation or hydroformylation.3 Selected recent examples are depicted in Figure 4. Feringa's group reported high ee's for the Cu catalyzed Michael addition of Grignard reagents to α,β-unsaturated esters,12a thioesters,12b,c and for selected cyclic enones.12d The preferred ligands were J001 and J004. The Rh/J001 was effective for the nucleophilic ring opening of various oxabicyclic substrates leading to interesting new tetrahydronaphthalene and cyclohexene derivatives.13a,b This reaction was scaled up to the kilogram scale.13c The Pd catalyzed opening of various cyclic anhydrides with Ph2Zn was described by Bercot and Rovis to occur with high ee's in presence of J001.14 Lorman et al.15 reported up to 95% ee for an intermolecular Heck reaction using a Pd/J001 catalyst.
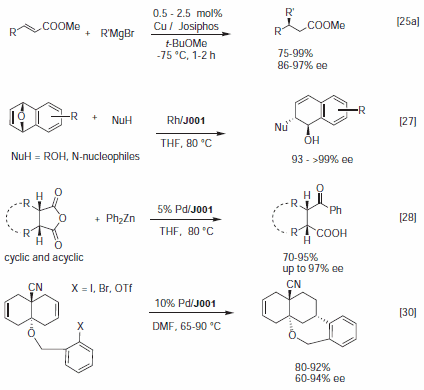
Figure 4.
Josiphos ligands were not only applied to enantioselective reactions but also were shown to be useful for Pd catalyzed coupling reactions with high activities. Hartwig's group reported that Pd/J009 (Product No. 88733 and 88734) complexes were effective catalysts for the amination of aryl halides or sulfonates with ammonia,16a coupling of aryl halides or sulfonates with thiols,16b and Kumada coupling of aryl or vinyl tosylates with Grignard reagents.16c
Walphos
Like Josiphos, Walphos ligands are modular but form 8‑membered metallocycles due to the additional phenyl ring attached to the cyclopentadiene ring.1 There are noticeable electronic effects but the scope of this ligand family is still under investigation; several derivatives are available from Solvias on a research scale. Walphos ligands show promise for various enantioselective hydrogenations and pertinent examples are depicted in Figure 1. Rh/Walphos catalysts gave good results for dehydro amino and itaconic acid derivatives1–3 (ee 92–95%, preferred ligands W001, W002, W004, W006 and W009) and of vinyl boronates4 (see Figure 1). Ru/Walphos complexes were highly selective for β-keto esters (ee 91‑95%, W001, W002, W003 and W005) and acetyl acetone (ee >99.5%, s/c 1000, W001).2,3 Recently, two novel transformations were reported to be catalyzed by Rh/Walphos complexes with high enantioselectivities (Figure 1): the 4+2‑addition of 4‑alkynals with an acryl amide by Tanaka and co-workers5 and the reductive coupling of enynes with α-keto esters by Krische's group.6
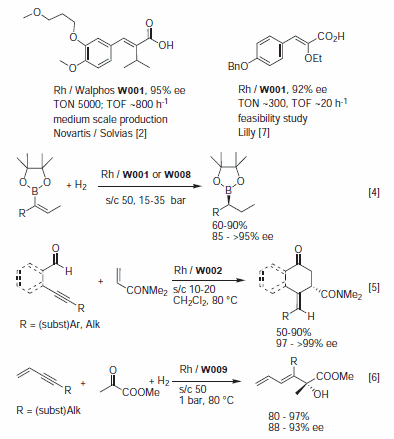
Figure 1.
The first industrial application was realized in collaboration with Speedel/Novartis for the hydrogenation of SPP100‑SyA, a sterically demanding α,β-unsaturated acid intermediate of the renin inhibitor SPP100.2 The process has already been operated on a multiton scale. Lilly's chemists7 developed a process of a PPAR agonist using the Rh-catalyzed asymmetric hydrogenation of (Z)-cinnamic acid as key step. A screen of over 250 catalysts and conditions revealed Rh-W001 as the most effective ligand, giving the product in 92% ee.
Taniaphos
Compared to Josiphos, the Taniaphos ligands developed by the Knochel group1 have an additional phenyl ring inserted at the side chain of the Ugi amine. Besides the two-phosphine moieties, the substituent at the stereogenic center can also be varied and, up to now, three generations of Taniaphos ligands with different substituents at the α-position have been prepared. Several Taniaphos ligands are being marketed by Solvias in collaboration with Umicore and selected derivatives are being produced on a multikilogram scale.
A variety of Taniaphos ligands are selective in a number of model hydrogenation reactions.1,2 Both the nature of two phosphine moieties, as well as the substituent and the configuration of the stereogenic center at the α-position, have strong effects on the catalytic performance, sometimes even on the sense of induction. Taniaphos complexes are highly active and stereoselective for the Rh catalyzed hydrogenation of methyl acetamido cinnamate (ee 94–99.5%) and dimethyl itaconate (91–99.5% ee), and in the Ru catalyzed hydrogenation of β-ketoesters (94–98% ee), cyclic β-ketoesters (85–94% ee, 99% de of anti-product, s/c up to 25,000) and 1,3‑diketones (97–99.4% ee, ~99% de of anti-product). Enamides are hydrogenated with 92–97% ee's but low TOFs (Rh/T021) and a β-dehydroacetamido acid with 99.5% (Rh/T003).
Recently, a variety of highly enantioselective transformations were described using Taniaphos complexes.3,4 Selected reactions are depicted in Figure 1. T001 was found to be selective for the Rh catalyzed nucleophilic ring opening of an azabicycle.3 Cu Taniaphos complexes were also effective for the reductive addition of aldehydes4a to methyl acrylate and of methyl ketones to methyl acrylate.4b
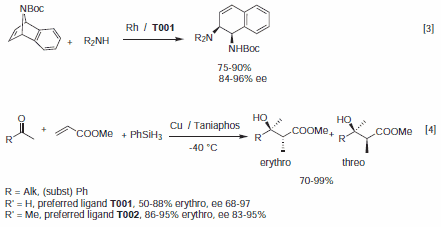
Figure 1.
Mandyphos (Ferriphos)
Ferriphos was first prepared by Ito and coworkers1 as a bidentate analog of PPFA. In 1998, Knochel2 developed a general synthesis for a highly modular ligand family, which was later called Mandyphos in which the PR'2 moieties and the substituents at the side chain can be used for fine tuning purposes. Selected Mandyphos derivatives are commercialized by Solvias in collaboration with Umicore. Even though the scope of this family is not yet fully explored, screening results3 indicate high enantioselectivities as well as high activity for several Mandyphos derivatives in the Rh catalyzed hydrogenation of dehydroamino acid derivatives (preferred ligand M004, 95 – >99% ee, s/c up to 20,000) and the Ru catalyzed hydrogenation of tiglic acid (M004, 97% ee). M004 was also the ligand of choice for the Rh catalyzed hydrogenation of various methyl 2‑furyl acrylates4 while Rh/Ferriphos was highly selective for Rh catalyzed ring opening of azabicycles with amines5 (see Figure 1). Several Mandyphos derivatives are being produced on a multikilogram scale.
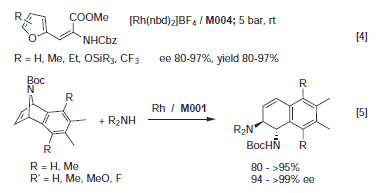
Figure 1.
Naud Catalyst
Complexes prepared in situ from RuCl2(PPh3)3 and chiral phosphine-oxazoline ligands (Naud catalysts) are effective catalysts for the hydrogenation of various aryl ketones with up to 99% ee's and substrate to catalyst ratios of 10,000–50,000. The reaction tolerates high substrate concentrations,1 and several derivatives are being produced on a multikilogram scale. A pilot process has been developed for the hydrogenation of 3,5‑bistrifluoromethyl acetophenone.2 The reaction was carried out twice on a 140 kg scale at 20 bar and 25 °C with substrate to catalyst ratios of 20,000 with an enantiomeric excess of >95%. After crystallization, (R)-3,5‑bistrifluoromethyl phenyl ethanol was obtained with an ee between 97.7 and 98.6% in 70% chemical yield (Figure 1). A feasibility study was reported for the reduction of a highly functionalized aryl ketone.3
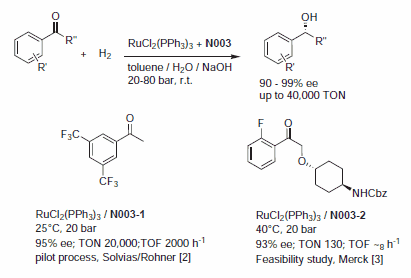
Figure 1.
Butiphane
In many respects the catalytic profile of the Butiphane ligands1 is rather similar to that of other phospholane diphosphines such as DuPhos and its many analogs.2 Up to now, the potential of the ligand has only been demonstrated in the Rh catalyzed hydrogenation of dehydroamino acid derivatives, enamides, and itaconates, achieving ee values of up to 98.7%.1
Reference (MeOBIPHEP)
Reference (Josiphos)
Reference (Walphos)
Reference (Taniaphos)
Reference (Mandyphos (Ferriphos))
Reference (Naud Catalyst)
Reference (Butiphane)
如要继续阅读,请登录或创建帐户。
暂无帐户?