Plasmid DNA Vaccines Manufacturing
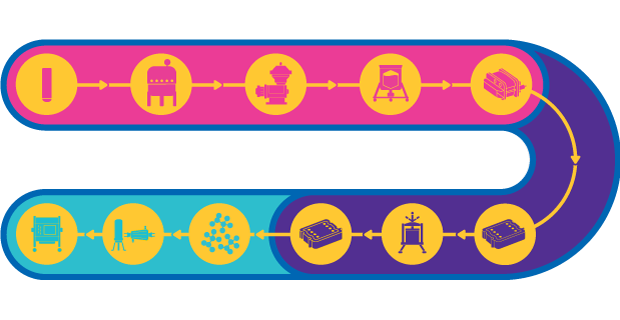
Plasmid DNA Vaccines Process
Plasmid DNA (pDNA) is an important component of viral vector therapies. These circular DNA molecules can be used as the therapeutic transgene, to code for the viral capsid or as the vaccine itself. DNA vaccines have been approved for use in animals and have been developed against the SARS-CoV-2 virus. pDNA is also used as the starting material for mRNA vaccines.
pDNA manufacturing presents several challenges. Production suffers from low productivity of microbial fermentation. Additionally, the purification process is complicated by the fact that the bacterial lysate is highly viscous and contains contaminants with properties similar to pDNA, leading to low resolution separation. The bacterial lysate can be highly viscous and contains contaminants with properties similar to pDNA leading to low resolution separation. These challenges can be overcome with use of an end-to-end platform consisting of advanced single-use technologies.
Related Resources
eBook: Plasmid DNA Downstream Process For Gene Therapy and Plasmid-based
DNA Vaccine Development
Webinar: Scalable Purification of Plasmid DNA for use in Vaccine Manufacturing
Technical Article: Downstream Applications for Plasmid DNA and Substrates
Technical Article: Manufacturing Strategies for mRNA Vaccines and Therapeutics
Technical Article: Plasmid DNA Purification
Webinar: Effective and Efficient Design of a Downstream Purification Process for Plasmid DNA
Application Note: Scalable Purification of Plasmid DNA – Strategies & Considerations for Vaccine and Gene Therapy Manufacturing
Application Note: Cell Harvest, Lysis, Neutralization & Clarification of Plasmid DNA
Application Note: Tangential Flow Filtration (UF/DF) of Plasmid DNA
Application Note: Chromatographic Purification of Plasmid DNA
Application Note: Sterilizing Grade Filtration Unit Operations for Plasmid DNA Processes
White Paper: Designing a Plasmid DNA Downstream Purification Process, for mRNA
Accelerate Time to Clinical while Increasing Upstream Productivity
pDNA presents several challenges that can be overcome with advanced single-use technologies for cell harvest and clarification. Centrifugation or microfiltration TFF (MF-TFF) is used to remove spent fermentation broth while depth filters have demonstrated excellent performance over a wide range of conditions for the clarification.
Achieve Yield and Efficiency Goals with Robust Impurity Removal
Anion exchange (AEX) has demonstrated robust clearance of proteins, RNA, gDNA and endotoxin. Plasmid isoforms, on the other hand, are very challenging to separate with ion exchange. In this case, HIC can be used and is well-placed following AEX due to the high salt eluate pool.
Maximize Downstream Recovery
Purification of pDNA is complex, requiring a combination of TFF and chromatography. TFF can be placed between the clarification and chromatography steps, where residual impurities can be washed through diafiltration and pDNA concentrated for chromatography. Both resins and membranes can be used for chromatographic purification. Resins offer flexible installations and good selectivity while membranes offer high binding capacity and flow rates.
Ensure Patient Safety
Once purified and formulated, pDNA must be sterile filtered to ensure patient safety. While this step may appear to be a relatively simple operation, filtration of pDNA can be challenging due to the large size of plasmids, high viscosity of the solution and bacterial retention for adjuvanted formulations. Many process parameters must be considered to optimize sterilizing grade filtration including salt concentration, plasmid size, purity, and plasmid concentration.
Downstream - Tangential Flow Filtration
Achieve yield, efficiency and pDNA recovery goals while ensuring robust impurity removal

Bioprocessing Liquid Cell Culture Media & Buffers
We offer the industry’s highest quality sterile filtered liquid capabilities, supplying ready-to-use cell culture media, buffers, CIP and SIP products from GMP facilities worldwide to optimize your biopharma production.
Downstream Chromatography
- Buffers & pH Ajusters
- Membrane Chromatography with Natrix® Q Pilot Chromatography Membranes
- Membrane Chromatography with Natrix® Q Recon Mini
- Capture an/or Polishing Chromatography with Fractogel® EMD DEAE Chromatography Resins
- Capture an/or Polishing Chromatography with Fractogel® EMD DMAE Chromatography Resins
Formulation, Sterile Filtration & Fill-Finish
Ensure patient safety with a reliable and robust sterile filtration process
To continue reading please sign in or create an account.
Don't Have An Account?