DTT-RO
Roche
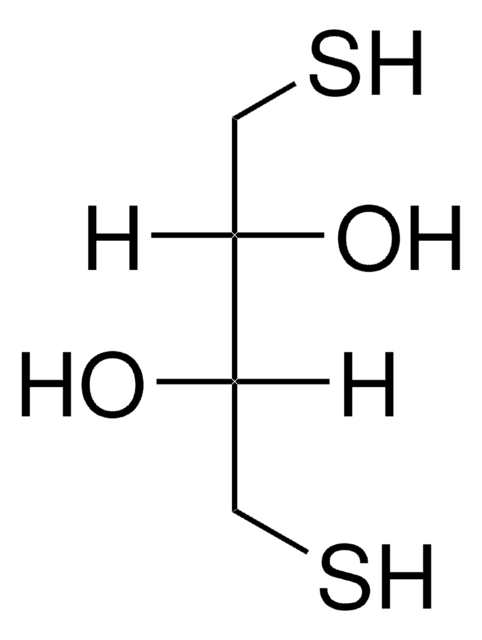
DTT
crystalline powder, =97% (Ellman′s reagent), Mr 154.3
Recommended Products
description
1,4-Dithiothreitol
Assay
97% (Ellman′s reagent)
form
crystalline powder
mol wt
Mr 154.3
reaction suitability
reagent type: reductant
packaging
pkg of 10 g (10708984001)
pkg of 2 g (10197777001)
pkg of 25 g (11583786001)
manufacturer/tradename
Roche
pH
5.1 (20 °C)
mp
41-44 °C (lit.)
storage temp.
2-8°C
SMILES string
O[C@H](CS)[C@H](O)CS
InChI
1S/C4H10O2S2/c5-3(1-7)4(6)2-8/h3-8H,1-2H2/t3-,4-/m1/s1
InChI key
VHJLVAABSRFDPM-QWWZWVQMSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Isolation, purification and characterization of proteins and enzymes
- Measurement of enzyme activities (reactivation of enzymes)
- Determination of disulfide groups in proteins and enzymes
- DNA extraction prior to amplification
Specifications
Sequence
Our DTT-preparation is optically inactive, i.e. it is the D,L-DTT.
Preparation Note
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Documents related to the products that you have purchased in the past have been gathered in the Document Library for your convenience.
Difficulty Finding Your Product Or Lot/Batch Number?
How to Find the Product Number
Product numbers are combined with Pack Sizes/Quantity when displayed on the website (example: T1503-25G). Please make sure you enter ONLY the product number in the Product Number field (example: T1503).
Example:
Additional examples:
705578-5MG-PW
PL860-CGA/SHF-1EA
MMYOMAG-74K-13
1000309185
enter as 1.000309185)
Having trouble? Feel free to contact Technical Service for assistance.
How to Find a Lot/Batch Number for COA
Lot and Batch Numbers can be found on a product's label following the words 'Lot' or 'Batch'.
Aldrich Products
For a lot number such as TO09019TO, enter it as 09019TO (without the first two letters 'TO').
For a lot number with a filling-code such as 05427ES-021, enter it as 05427ES (without the filling-code '-021').
For a lot number with a filling-code such as STBB0728K9, enter it as STBB0728 without the filling-code 'K9'.
Not Finding What You Are Looking For?
In some cases, a COA may not be available online. If your search was unable to find the COA you can request one.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service