Vitamin D and Prevention of Human Disease
Vitamin D and Prevention of Human Disease
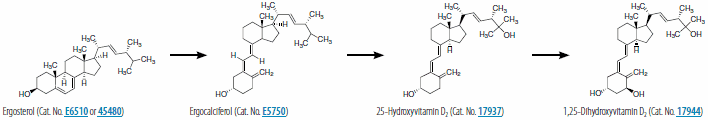
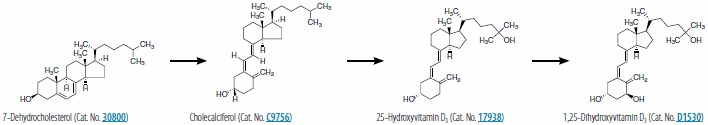
Vitamin D2 (ergocalciferol) is naturally synthesized from ergosterol by invertebrates, fungi, and plants in response to ultraviolet B irradiation, while vitamin D3 synthesis (cholecalciferol) is uniquely initiated in the skin of vertebrates. During sun exposure, ultraviolet B photons are absorbed by 7-dehydrocholesterol, which is found within the plasma membranes of epidermal and dermal skin layers. This reaction yields an unstable derivative of 7-dehydrocholesterol, named precholecalcitrol, which rapidly rearranges to vitamin D3. Vitamin D binding protein (DBP) is a carrier protein responsible for drawing vitamin D3 from the plasma membrane into the dermal capillaries within the extracellular space. Vitamin D3 may be commercially synthesized from cholesterol extracted from the lanolin of sheep wool. The extracted cholesterol is converted to 7-dehydrocholesterol and then treated with UV light to produce vitamin D3. Both vitamin D2 and D3 are commercially used in fortified foods and supplements.
Both forms of vitamin D undergo two hydroxylations to form the active metabolite, 1,25-dihydroxyvitamin D (1,25(OH)2D), termed calcitriol (1,25(OH)2D3) when specifically referring to the synthesis from vitamin D3. The first hydroxylation occurs in the liver where D-25-hydroxylase, encoded by CYP2R1 (cytochrome P450, family 2, subfamily R, polypeptide) converts vitamin D3 into 25-hydroxyvitamin D3 (calcidiol or 25(OH)D3). With the assistance of DBP, 25-hydroxyvitamin D3 circulates into a renal tubule, where a second hydroxylation by 25-hydroxyvitamin D-1 α-hydroxylase, encoded by CYP27B, yields 1,25-dihydroxyvitamin D3 (1,25(OH)2D3). This reaction is inhibited by free calcium, inorganic phosphate, and the end product (1,25(OH)2D), and stimulated by parathyroid hormone (PTH), a regulator of 25-hydroxyvitamin D-1 α-hydroxylase. Figures 1 and 2 show the synthesis pathway of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3. 1,25(OH)2D is responsible for the intestinal transport of calcium, renal calcium absorption, bone formation and maintenance, insulin secretion, and blood pressure regulation. To initiate these biological processes, 1,25(OH)2D binds with its high-affinity vitamin D receptor (VDR), a ligand-activated transcription factor, which complexes with retinoid X receptor (RXR) to form a hetereodimer, resulting in vitamin D response element recognition and gene transcription related to these biological processes.
In the intestine, binding of the hetereodimer complex regulates the transcription of the calcium binding protein, calbindin, which assists in the transfer of calcium across the cell membrane and into the bloodstream. The 1,25(OH)2D assisted regulation of calbindin expression appears to control the intracellular calcium flux within insulin-secreting islet cells. 1,25(OH)2D is also critical for osteoblastic bone formation and osteoclastic maintenance. In the osteoblast, the hetereodimer induces the expression of receptor activator NF-κΒ ligand (RANKL). The binding of RANKL to the RANK receptor unleashes a signaling cascade resulting in differentiation and osteoclast growth. In the parathyroid glands, 1,25(OH)2D is involved in the suppression of PTH gene transcription, the regulation of VDR concentration, and parathyroid response to calcium. The correlation between vitamin D deficiency and high blood pressure may be attributed to the role of 1,25(OH)2D as a negative endocrine regulator of the renin-angiotensin system. An experimental study showed that VDR knock-out mice experienced high levels of renin expression and angiotensin production resulting in hypertension.
In addition to the involvement of VDR in these biological processes, VDR is required for the β-catenin (cadherin-associated protein) assisted induction of hair follicle formation in adult epidermis. Palmer et al., demonstrated in transgenic mice constructed with a 4-hydroxy-Tamoxifen (4OHT) inducible form of stabilized β-catenin under the control of the keratin 14 promoter (K14ΔNβ- cateninER) that a vitamin D analog inhibits β-catenin-induced hair follicle tumor formation. Figure 3, A-C compares the tails of vitamin D analog (EB1089) treated and non-treated mice and stained tail sections of wild type and K14ΔNβ-cateninER transgenic mice. Palmer et al., also reported in the absence of VDR, β-catenin induces human tumors characterized as infiltrative basal cell carcinomas. Figure 3, D and E, shows stained human skin sections of trichofolliculoma and basal cell carcinoma labeled with β-catenin and VDR. The experimental study concluded that VDR is a transcriptional effector of the Wnt pathway that promotes hair follicle differentiation and modulates Wnt-induced tumor formation. This investigation is an example of recent research providing a deeper understanding of the involvement of vitamin D in cancer-related mechanisms.
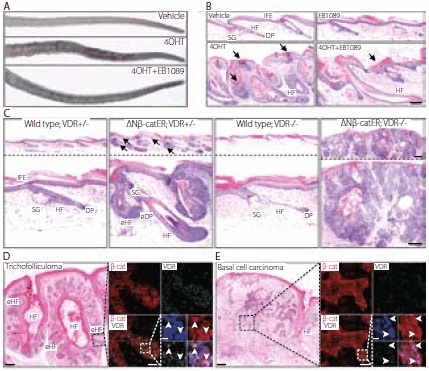
Figure 3.VDR modulates β-catenin induced skin tumors. (A, B) K14ΔNβ-cateninER (D4) transgenic mice were treated with vehicle, 4-hydroxy-Tamoxifen (4OHT), EB1089 or 4OHT+EB1089 for 21 days. (A) Macroscopic appearance of tails. (B) Hematoxylin and eosin (H&E) stained tail skin sections. Arrows indicate parakeratosis and increased cornified layers. (C) H&E stained tail skin sections of wild type and K14ΔNβ-cateninER (D4) transgenic mice that were heterozygous (+/–) or homozygous (–/–) null for VDR, following 4OHT treatment for 21 days. Arrows indicate ectopic hair follicle formation. eHF: ectopic hair follicles; DP: dermal papilla; eDP: ectopic dermal papilla. (D, E) Human trichofolliculoma (D) and infiltrative basal cell carcinoma (E). Serial sections were stained with H&E or immunolabelled for β-catenin (red) and VDR (green) with Hoescht counterstain (blue). Immunolabelling corresponds to boxed regions of H&E stained sections. Arrowheads show nuclear β-catenin and VDR. Scale bars: 100 μm (B–E), 50 μm (inserts in d, e), 10 μm (Hoescht staining inserts d,e). Copyright © Palmer, H.G. et al., PLoS ONE, 3(1), e1483. This image is from an open-access article distributed under the terms of the Creative Commons Attribution License
Studies have linked vitamin D deficiency to colon, prostate, breast, ovarian, lymphoma, and other devastating cancer types. It is has been stated that increasing the population’s average circulating 25(OH)D level to ~50 ng/mL would prevent ~58,000 new cases of breast cancer and ~49,000 cases of colorectal cancer each year. However, upper level dose limits are critical since effective therapeutic levels may produce hypercalcemia. This information is based on observational studies combined with randomized trials. Vitamin D guidelines for cancer prevention have focused on the levels of 25(OH)D rather than 1,25(OH)2D in the bloodstream, since the production of 1,25(OH)2D is tightly controlled by the kidneys.
An enhanced level of vitamin D through sun exposure or dietary intake does not result in a measurable increase of 1,25(OH)2D production but does result in a measurable increase of 25(OH) D concentration. The kidney is not the only location of 25(OH) D hydroxylation; a wide variety of tissues, including skin, breast, colon, lung, and brain have the enzymatic ability to metabolize 25(OH)D into 1,25(OH)2D. It has been suggested that raising levels of 25(OH)D in the bloodstream provides enough 25(OH)D substrate to enable various tissue types to use locally synthesized 1,25(OH)2D for protection against uncontrollable cell growth and maturation, and malignancy risk.
Cell growth and maturation control may be attributed to the antiangiogenic properties of vitamin D. Mantell, et al., showed 1,25(OH)2D treatment on “activated” endothelial cells significantly inhibited vascular endothelial growth factor (VEGF)-induced endothelial cell sprouting and elongation, a required stage in the angiogenic process. An additional in vivo study showed 1,25(OH)2D treatment produced tumors were less vascularized then tumors formed in mice without 1,25(OH)2D treatment.
Previous research findings regarding vitamin D and cancer have led to the creation of a new cancer etiology model, referred to as DINOMIT (disjunction, initiation, natural selection, overgrowth, metastasis, involution, and transition). The model describes the loss of communication between cells as the driver in cancer development. This new model differs significantly from the carcinogenesis and cancer stem cell models. Cedric Garland, UCSD said “In this new model, we propose that this loss may play a key role in cancer by disrupting the communication between cells that is essential to healthy cell turnover, allowing more aggressive cancer cells to take over.” When there are adequate vitamin D levels, cells adhere, communicate, and act as mature epithelial cells but with inadequate vitamin levels, they may lose their stickiness along with their identity as differentiated cells, and revert to a stem cell-like state. Garland further stated “vitamin D may halt the first stage of the cancer process by re-establishing intercellular junctions in malignancies having an intact vitamin D receptor”.
Although the DINOMIT model and other scientific reports presented in this article provide a strong explanation for the beneficial value of vitamin D, additional research and long-term clinical studies are needed to fully understand the impact of vitamin D in human disease prevention.
To continue reading please sign in or create an account.
Don't Have An Account?